Current treatments can slow progression of heart failure, but do not address the underlying issues, including specific problems that cause systolic heart failure. In this condition, the heart doesn’t contract vigorously enough in pushing blood into the body’s circulation. But findings at nanometer and millisecond scales, based upon experimental data collected at the U.S. Department of Energy’s Advanced Photon Source (APS) may help improve design of therapies directed at motor proteins to rescue failing hearts.
The heart muscle contractions that pump blood are generated by interactions between actin and myosin. These motor proteins power movement at the molecular level by converting the molecule ATP into energy. Earlier research in the lab of Michael Regnier, University of Washington (UW) professor of bioengineering, had shown that dATP, a natural variant of ATP, can be used to promote stronger heart function.
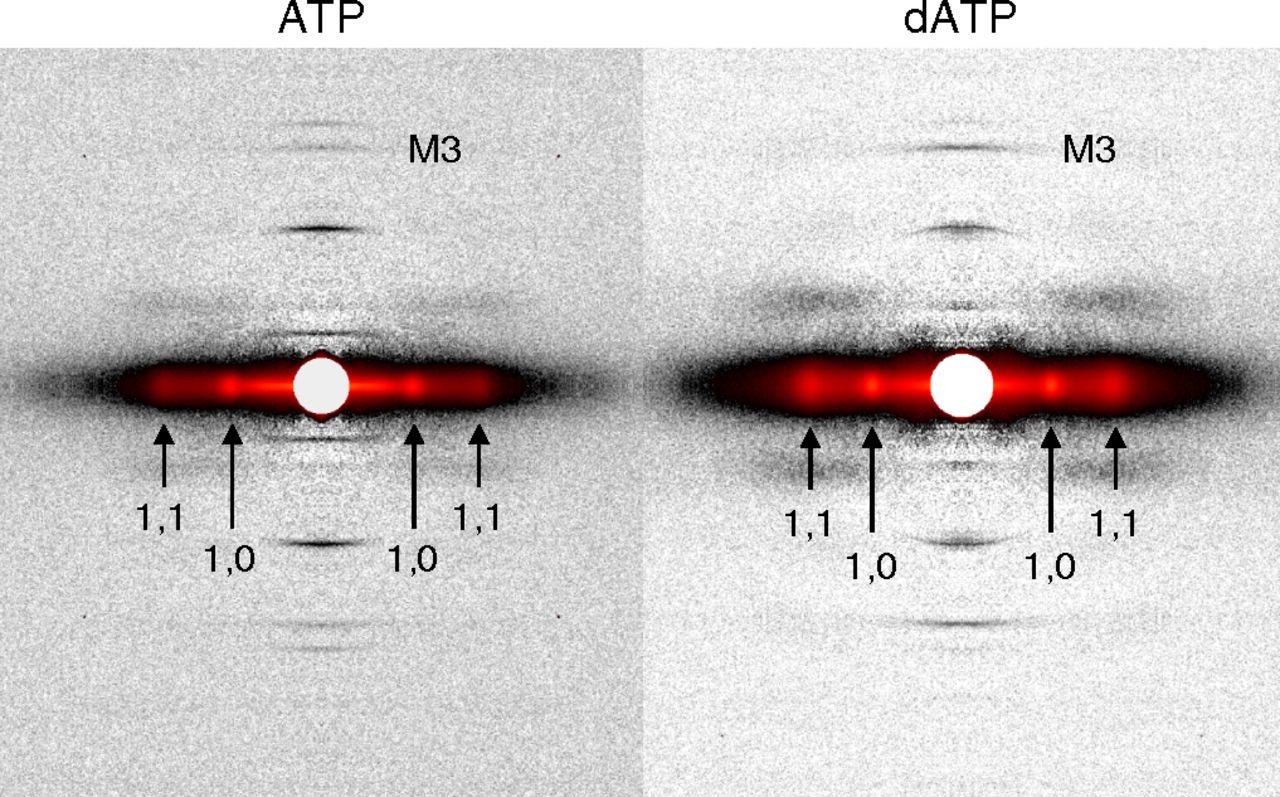
However, there remains a pressing need for data to explain why dATP helps to increase contractile force in heart disease. A new study headed by Regnier, who is a researcher at the UW Medicine Institute for Stem Cell and Regenerative Medicine and director of the Center for Translational Muscle Research, offers new insights, with unprecedented precision, about the nature of dATP. The results, which were detailed in the journal Proceeding of the National Academy of Sciences of the United States of America, were derived from synchrotron x-ray diffraction experiments utilizing the small-angle x-ray scattering instrument on the Biophysics Collaborative Access Team beamline 18-ID at the APS (Fig. 1). The first author of the paper is bioengineering graduate student Joseph D. Powers. (The APS is an Office of Science user facility at Argonne National Laboratory.)
The research team, which includes scientists from the University of Washington, the University of California, San Diego, and the Illinois Institute of Technology, hypothesized that dATP plays the role of matchmaker.
“Myosin and actin are attracted to each other by electrostatic charges,” said Regnier, referring to the electrical force that brings two oppositely charged objects together. “Our hunch was that dATP enhances the initial attraction between these two critical muscle proteins and thereby leads to stronger contractions.”
By developing new experimental and computational approaches, the research team showed how small changes in protein structure translate to improved performance at multiple scales – the whole muscle, muscle cell, and the molecular levels. Their findings revealed a more holistic picture of the mechanisms behind dATP-augmented cardiac contractile force.
Regnier explained the main takeaways from the study: “A key finding was that small changes on the surface of myosin occur when dATP is used enhance its ability to bind with actin. This suggests the structure of myosin can be optimized and opens the door to possible new therapies to improve contraction of weakened heart muscle. This report offers a novel approach and a new standard for design and characterization of future cardiac therapeutics for systolic heart failure.”
See: Joseph D. Powers1,2, Chen-Ching Yuan1, Kimberly J. McCabe2, Jason D. Murray1, Matthew Carter Childers1, Galina V. Flint1, Farid Moussavi-Harami1, Saffie Mohran1, Romi Castillo1, Carla Zuzek1, Weikang Ma3, Valerie Daggett1, Andrew D. McCulloch2, Thomas C. Irving3, and Michael Regnier1, “Cardiac myosin activation with 2-deoxy-ATP via increased electrostatic interactions with actin,” Proc. Natl. Acad. Sci. USA, published on line May 20, 2019.
DOI: 10.1073/pnas.1905028116
Author affiliations: 1University of Washington, 2University of California, San Diego, 3Illinois Institute of Technology
Correspondence: *j2powers@ucsd.edu, ** mregnier@uw.edu
This work was supported by National Institutes of Health (NIH) Grants R01 HL128368 and R56 AG055594 (to M.R.), NIH Grant U01 HL122199 (to M.R. and A.D.M.), NIH Grant T32-HL007312 (to J.D.P.), a Sackler Scholars Program Fellowship in Integrative Biophysics (to C.-C.Y.), NIH Grant T32-HL105373 (to K.J.M.), NIH Grant K08 HL128826 (to F.M.-H.), and NIH Grant T32-EB1650 (to J.D.M.). This project was supported by grant 9 P41 GM103622 from the National Institute of General Medical Sciences of the NIH. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by the Argonne National Laboratory under Contract DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
