 Results of research carried out at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS) may pave the way to improvements in industrial processes based on solvent extraction, which is used in the mining and refinement of technologically important rare earths. The results were published in the journal Physical Review Letters.
Results of research carried out at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS) may pave the way to improvements in industrial processes based on solvent extraction, which is used in the mining and refinement of technologically important rare earths. The results were published in the journal Physical Review Letters.
Rare earths such as lanthanides, which are elements in the range of atomic number 57 to 71, are not actually rare. They exist in large quantities in the world, but are only found in the form of trace amounts in rocks. Since rare earths are important for a variety of applications (e.g., electronics) their extraction is a major mining-related industry.
A common process by which rare earths are extracted involves dissolving rocks in acids, then shaking up the solution with an organic solvent and a surfactant. Under the right conditions, the desired ions move out of the aqueous phase and into the organic solvent. This is known as “liquid-liquid extraction” or “solvent extraction,” and is conducted on a large scale by the mining industry. This process also separates heavier lanthanides from lighter lanthanides present in the same solution, because the heavier lanthanides separate more easily. While this fact is known and exploited in industrial separations processes, the nanoscale mechanisms of the separation process are not well understood.
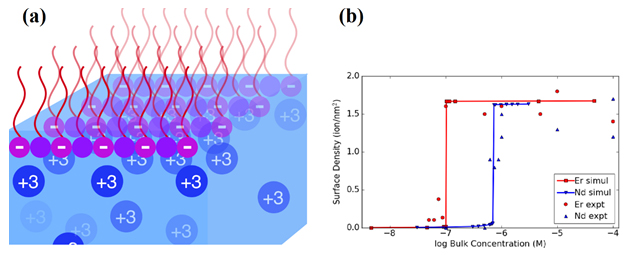
New light has been shined on these mechanisms thanks to x-ray diffraction experiments carried out at the ChemMatCARS 15-ID-B,C,D x-ray beamline at the APS (the APS is an Office of Science user facility at Argonne National Laboratory). Employing synchrotron x-ray fluorescence measurements, researchers from Northwestern University and The University of Chicago revealed that a heavier lanthanide segregates to a surfactant-laden interface at much lower concentrations compared to a lighter lanthanide.
Furthermore, the concentration-dependent onset of surface segregation happens quite suddenly at a specific solution concentration (reminiscent of a phase transition, such as that between ice and water). The authors explain this unexpected behavior using computer simulations based on electrostatic interactions. In solutions that contain two lanthanides at the same time, the heavier lanthanide dominates at the interface, displacing the lighter lanthanide.
Together, these results imply that ionic selectivity during separation happens at the interface rather than in the bulk or during dynamic processes such as stirring or mixing. By revealing the nanoscale processes responsible, such studies may pave the way to improvements in industrial extraction processes.
See: Mitchell Miller1, Yihao Liang1, Honghao Li1, Miaoqi Chu1, Sangjun Yoo1, Wei Bu2, Monica Olvera de la Cruz1, and Pulak Dutta1*, “Electrostatic Origin of Element Selectivity during Rare Earth Adsorption,” Phys. Rev. Lett. 122, 058001 (2019). DOI: 10.1103/PhysRevLett.122.058001
Author affiliations: 1Northwestern University, 2The University of Chicago
Correspondence: * pdutta@northwestern.edu
M.M., M. C., S.Y., and P.D. were supported by National Science Foundation (NSF) Grant No. DMR- 362 1612876. Y.L., H.L., and M.O. were supported by NSF Grant No. DMR-1611076. ChemMatCARS is supported by the U.S. National Science Foundation, Grant No. CHE- 367 1346572. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
