The original University College London press release by Bex Caygill can be read here.
Tiny, disordered particles of magnesium chromium oxide may hold the key to new magnesium battery energy storage technology, which could possess increased capacity compared to conventional lithium-ion batteries, find researchers who studied the material utilizing ultra-bright x-ray beams from the U.S. Department of Energy’s Advanced Photon Source (APS).
The study, published in the journal Nanoscale, reports a new, scalable method for making a material that can reversibly store magnesium ions at high-voltage, the defining feature of a cathode. While it is at an early stage, the researchers say it is a significant development in moving towards magnesium-based batteries. To date, very few inorganic materials have shown reversible magnesium removal and insertion, which is key for the magnesium battery to function.
“Lithium-ion technology is reaching the boundary of its capability, so it's important to look for other chemistries that will allow us to build batteries with a bigger storage capacity and a slimmer design,” said co-lead author Dr. Ian Johnson (University College London, Chemistry).
“Magnesium battery technology has been championed as a possible solution to provide longer-lasting phone and electric car batteries, but getting a practical material to use as a cathode has been a challenge.”
One factor limiting lithium-ion batteries is the anode. Low-capacity carbon anodes have to be used in lithium-ion batteries for safety reasons, as the use of pure lithium metal anodes can cause dangerous short circuits and fires.
In contrast, magnesium metal anodes are much safer, so partnering magnesium metal with a functioning cathode material would make a battery smaller and store more energy.
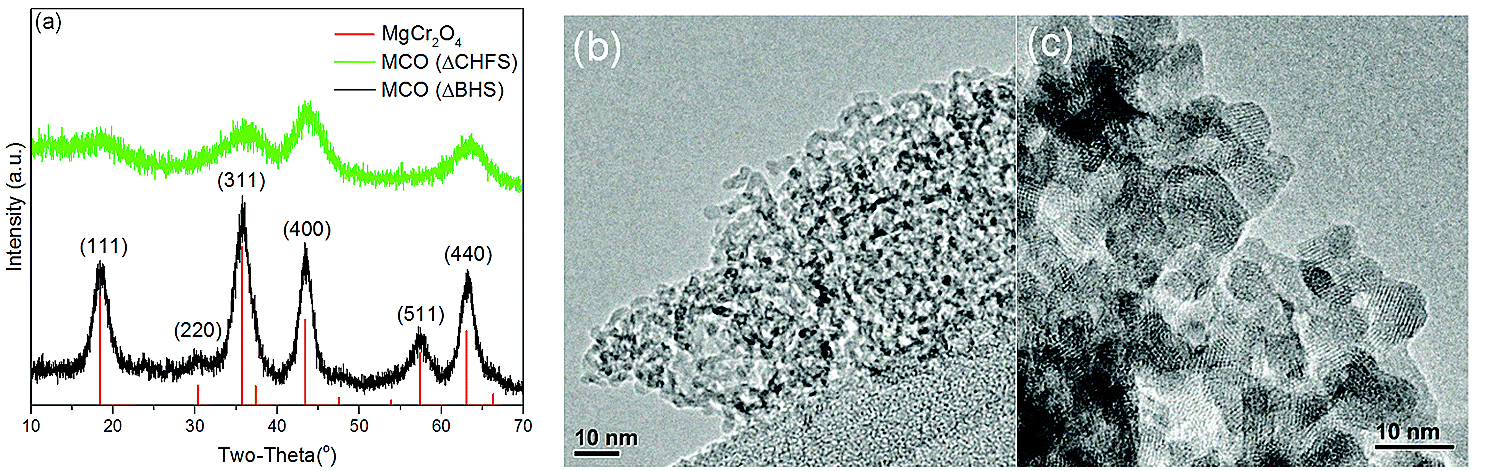
Previous research using computational models predicted that magnesium chromium oxide (MgCr2O4) could be a promising candidate for Mg battery cathodes. Inspired by this work, University College London researchers produced ~5-nm-size, poorly-crystalline particles of disordered magnesium chromium oxide material using a very rapid, relatively low-temperature flow process. This material was cast on a steel mesh to produce working a electrode that was tested electrochemically. Collaborators at the University of Illinois at Chicago subsequently compared its magnesium activity with an ordered magnesium chromium oxide material of ~7-nm size.
To see the structural and chemical changes when the two materials were tested for magnesium activity in a cell, the researchers and colleagues from Argonne National Laboratory and Pusan National University (Republic of Korea) employed a range of different techniques including x-ray powder diffraction (Fig. 1) at X-ray Science Division (XSD) beamline 11-ID-B at the APS (an Office of Science user facility at Argonne National Laboratory); soft x-ray absorption spectroscopy measurements at XSD beamline 4-ID-C; and x-ray absorption spectroscopy at the Materials Research Collaborative Access Team 10-BM-B beamline, also at the APS; and cutting-edge electrochemical methods.
The two types of crystals behaved very differently, with the disordered particles displaying reversible magnesium extraction and insertion, compared to the absence of such activity in larger, ordered crystals.
“This suggests the future of Mg batteries might lie in the use of disordered and unconventional structures, which is an exciting prospect and one we've not explored before as usually disorder gives rise to issues in battery materials. It highlights the importance of seeing if other structurally defective materials might give further opportunities for reversible battery chemistry,” said Professor Jawwad Darr (University College London, Chemistry).
“We see increasing the surface area and including disorder in the crystal structure offers novel avenues for important chemistry to take place compared to ordered crystals. Conventionally, order is desired to provide clear diffusion pathways, allowing cells to be charged and discharged easily - but what we've seen suggests that a disordered structure introduces new, accessible diffusion pathways that need to be further investigated," said Professor Jordi Cabana (University of Illinois at Chicago).
These results are the product of an exciting new collaboration between UK and US researchers. University College London and the University of Illinois at Chicago intend to expand their studies to other disordered, high surface area materials, to enable further gains in magnesium storage capability and develop a practical magnesium battery.
Funding for the project was provided by the Joint Center for Energy Storage Research, a US Department of Energy Innovation Hub, and the JUICED Energy Hub by the Engineering and Physical Sciences Research Council.
See: Linhua Hu1,2, Ian D. Johnson3, Soojeong Kim2, Gene M. Nolis2,3, John W. Freeland2, Hyun Deog Yoo3, Timothy T. Fister2,3, Liam McCafferty2, Thomas E. Ashton3, Jawwad A. Darr3**, and Jordi Cabana1,2*, “Tailoring the electrochemical activity of magnesium chromium oxide towards Mg batteries through control of size and crystal structure,” Nanoscale, Advanced Article, (December 2019). DOI: 10.1039/C8NR08347A
Author affiliations: 1University of Illinois at Chicago, 2Argonne National Laboratory, 3University College London, 3Pusan National University
Correspondence: *jcabana@uic.edu, **j.a.darr@ucl.ac.uk
This work was supported as part of the Joint Center for Energy Storage Research, an Energy Innovation Hub funded by the U.S. Department of Energy (DOE) Office of Science-Basic Energy Sciences, under contract DE-AC02-05CH11231. I.D.J., T.E.A., L.M., and J.A.D. would like to thank the EPSRC for funding the JUICED Energy Hub (EP/R023662/1) and the ELEVATE (Electrochemical Vehicle Advanced Technology; P/M009394/1) project. I.D.J. would also like to thank the Materials Modelling and Molecular Doctoral Training Centre (EP/G036675/1), and the STFC for providing funding support for travel within the collaboration (STFC/MDC Futures Early Career Award, ST/N002385/1). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
