Imagine a sponge that could soak up a thousand times its own volume in water. Now imagine how effective that sponge would be if it could store hydrogen instead of water, giving researchers an alternative to compressed air cylinders for storing the gas.
Palladium, a precious metal closely related to platinum, is that sponge. Unlike any other element, it takes up hydrogen at room temperature and pressure. In a recent study, scientists at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have gained new insight into how this uptake of hydrogen occurs, realized how it impacts the atomic structure of the palladium, and identified key properties of how this form of hydrogen storage could work in the future.
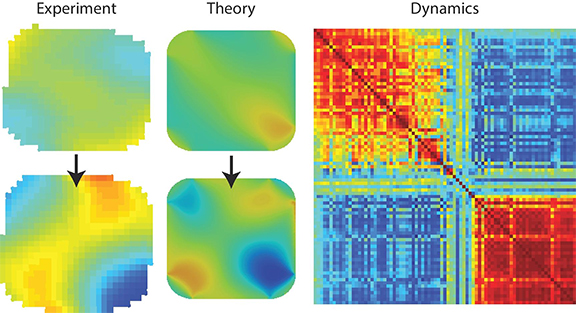
When hydrogen is cycled into the palladium nanoparticles, it alters and degrades the particles’ structure over time due to strain. “It’s like trying to put your foot in too small of a shoe,” said Argonne postdoctoral researcher Andrew Ulvestad, who was the study’s first author.
For more see the Argonne press release by Jared Sagoff.
See: A. Ulvestad, M.J. Welland, S.S.E. Collins, R. Harder, E. Maxey, J. Wingert, A. Singer, S. Hy, P. Mulvaney, P. Zapol, O.G. Shpyrko, "Avalanching strain dynamics during the hydriding phase transformation in individual palladium nanoparticles," Nat. Commun. 6, 10092-1 (2015). DOI: 10.1038/ncomms10092
This research used resources of the Advanced Photon Source, a U.S. Department of Energy Office of Science User Facility operated for the U.S. Department of Energy Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.