The original University of California, San Diego press release by Daniel Kane can be read here.
Scientists using two synchrotron x-ray light sources including the U.S. Department of Energy’s Advanced Photon Source (APS) have an explanation for the cause of performance-reducing “voltage fade” that currently plagues a promising class of cathode materials called lithium-rich NMC (nickel magnesium cobalt) layered oxides.
These cathode materials have garnered considerable attention over the years as promising components for better rechargeable batteries for electric vehicles.
After a battery goes through a series of charge-discharge cycles, its voltage fades and the amount of energy it can hold, and release later for use, also fades. The new research explains why this happens in lithium-rich NMC cathode materials. In particular, the researchers identified nanoscale defects or dislocations in lithium-rich NMC cathode materials as the batteries charged at a range of voltages going up to 4.7 volts.
"The dislocations are extra atomic layers that don’t fit into the otherwise perfectly periodic crystal structure,” said Andrej Singer, the lead author of the article on this study that was published in the journal Energy Research, who performed this work as a postdoctoral researcher at UC San Diego. “Discovering these dislocations was a big surprise: if anything, we expected the extra atomic layers to occur in a completely different orientation,” said Singer, who is now on the faculty at Cornell University. By combining experimental evidence with theory, the research team concluded that the nucleation of this specific type of dislocation results in voltage fade.
Knowing the origin of voltage fade, the team showed that heat-treating the cathode materials eliminated most of the defects and restored the original voltage. They put the heat-treated cathodes into new batteries and tested them at a range of voltages going up to 4.7 volts, demonstrating that the voltage fade had been reversed.
While the heat treating approach to reversing the defects is labor intensive and not likely to scale, the physics and materials science-based approach to characterizing and then addressing the nano-scale defects offers promise for finding new solutions to the voltage fade problem.
“Our paper is mainly about unlocking the mystery of the dislocations that cause voltage fade in lithium-rich NMCs. We don’t have a scalable solution yet to solving the voltage fade problem in lithium-rich NMCs, but we are making progress,” said UC San Diego nanoengineering professor Shirley Meng. She and UC San Diego Physics professor Oleg Shpyrko are the senior authors on the new Nature Energy paper.
“One of the most serious problems for lithium-rich NMC cathode materials is voltage fade,” said paper author Minghao Zhang, a recent graduate of the nanoengineering Ph.D. program at UC San Diego Jacobs School of Engineering, where he is now a postdoctoral researcher.
Voltage fade reduces the energy density of the battery, which in turn limits the practical applications of these materials despite their high energy density in the initial charge-discharge cycles.
“Our work for the first time clearly demonstrates that defect generation and defect accumulation in the structure of lithium-rich NMC materials are the origin of voltage fade,” said Zhang. “Based on this explanation, we designed a heat treatment regime and then showed that the heat treatments removed the defects in the bulk structure and restored the battery output voltage.”
“Engineering solutions have to be based on solid science. If you don’t know what’s going on, then your mitigation strategies are less effective. And I think that is what has hindered this material,” said UC San Diego nanoengineering professor Shirley Meng, referring to the long-standing lack of clarity on what is happening at the nanoscale that is causing the voltage fade in these promising cathode materials.
Meng, Shpyrko, and their respective labs and collaborators are uniquely adept at imaging, characterizing and calculating what is happening to batteries, at the nanoscale, while they are charging. Their combined expertise allows the team to glean unprecedented insights from x-ray imaging data of batteries while they are charging.
“Being able to directly image the structure of materials and devices under operating conditions and with nanoscale resolution is one of the grand challenges in our quest to design and discover new functional materials,” said UC San Diego physics professor Oleg Shpyrko. “Our group’s efforts in developing novel x-ray imaging techniques are targeted towards fundamental understanding and ultimately control of defect formation. Our in-operando imaging studies indicate novel ways of mitigating voltage fade in next-generation energy storage materials.”
In the Nature Energy paper, the authors write: “We directly capture the nucleation of a dislocation network in primary nanoparticles of a high capacity LRLO material [a lithium-rich NMC cathode] during electrochemical charge. Based on the discovery of defect formation and first principles calculations, we identify the origin of the voltage fade, allowing us to design and experimentally demonstrate an innovative treatment to restore voltage in LRLO.”
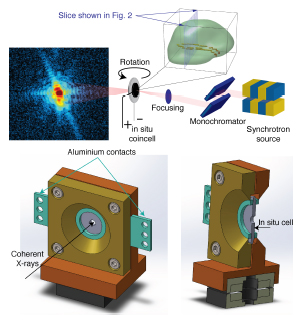
The in situ Bragg coherent diffractive imaging technique, performed at the X-ray Science Division 34-ID-C x-ray beamline at the Argonne National Laboratory APS (an Office of Science user facility) and at the P10 beamline of the PETRA III (Deutsches Elektronen Synchrotron, DESY, Germany) x-ray light source, allowed the researchers to directly image the interior of a nanoparticle during battery charge. The team’s analyses and reconstructions of these data offer unprecedented insights into what is actually happening while batteries are charging. The researchers performed a number of observational studies while battery materials were charging across a range of voltages going from 4 volts up to 4.7 volts. At 4.4 volts, the researchers identified a series of defects including edge, screw and mixed dislocations. The researchers also studied currently-commercialized non-lithium-rich NMC materials and found defects, but significantly fewer; and no new defects occurred above 4.2 volts in the non-lithium-rich NMC materials.
“With this publication, we are hoping to open up a new paradigm for materials scientists to rethink how to design and optimize this class of materials for energy storage. It still requires a lot more work and many contributions from the field to finally resolve the problem,” said Meng.
The research described in the Nature Energy paper could eventually lead to new cathode materials for solid state batteries. Many researchers, including Meng, consider solid state batteries to be one of the most promising future battery approaches. Lithium-rich NMC cathodes, for example, operate at high voltage and therefore could eventually be paired with solid state electrolytes, which also operate at high voltage. Much of the interest in solid state batteries comes from the fact that solid state electrolytes are believed to be safer than the traditional liquid electrolytes used in lithium-ion rechargeable batteries.
See: A. Singer1*, M. Zhang1, S. Hy1, D. Cela1, C. Fang1, T. A. Wynn1, B. Qiu2, Y. Xia2, Z. Liu2, A. Ulvestad3, N. Hua1, J. Wingert1, H. Liu1, M. Sprung4 A. V. Zozulya4‡, E. Maxey3, R. Harder3, Y.S. Meng1*, and O. G. Shpyrko1*, “Nucleation of dislocations and their dynamics in layered oxides cathode materials during battery charging,” Nat. Energy, 3, 641 (2018). DOI: 10.1038/s41560-018-0184-2
1University of California San Diego, 2Chinese Academy of Sciences, 3Argonne National Laboratory, 4Deutsches Elektronen-Synchrotron DESY *Present address: Cornell University, ‡Present address: European XFEL GmbH
Correspondence: *shirleymeng@ucsd.edu, **oshpyrko@physics.ucsd.edu
X-ray imaging was supported by the U.S. Department of Energy (DOE) Office of Science-Basic Energy Sciences, under contract DE-SC0001805 (A.S., D.C., J.W., N.H. and O.G.S.). S.H., C.F., M.Z., H.L., and Y.S.M. acknowledge support on the materials synthesis, electrochemical, and materials characterization from the NorthEast Center for Chemical Energy Storage (NECCES), an Energy Frontier Research Center funded by the U.S. DOE Office of Science-Basic Energy Sciences under Award no. DE-SC0012583. The sample exchanges and collaborations between UCSD and NIMTE are made possible with the support from Office of Vehicle Technology of the U.S. DOE under the Advanced Battery Materials Research Program. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. The authors thank Ross Harder (XSD), and all of the staff at Argonne National Laboratory and the Advanced Photon Source for their assistance with the synchrotron studies. PETRA III at DESY is a member of the Helmholtz Association. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
