The original Purdue University press release by Emil Venere can be read here.
A team of researchers utilizing two U.S. Department of Energy synchrotron x-ray sources, including the Advanced Photon Source (APS), have shown how to shuttle lithium ions back and forth into the crystal structure of a quantum material, representing a new avenue for research and potential applications in batteries, “smart windows,” and brain-inspired computers containing artificial synapses.
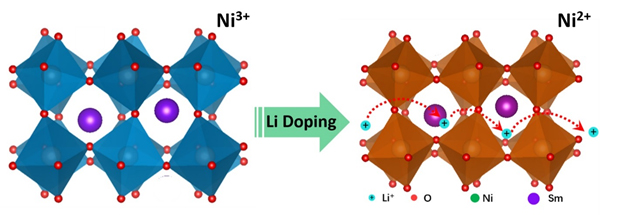
The investigation centers on the material samarium nickelate, which is a quantum material, meaning its performance taps into quantum mechanical interactions. Samarium nickelate is in a class of quantum materials classified as strongly correlated electron systems, which have exotic electronic and magnetic properties. Findings are detailed in a paper published in the Proceedings of the National Academy of Sciences of the United States of America.
The researchers from Purdue University; Rutgers, The State University of New Jersey; Argonne National Laboratory; the Massachusetts Institute of Technology; Brookhaven National Laboratory; Osaka University (Japan),;and the University of Georgia “doped” the material with lithium ions, meaning the ions were added to the material’s crystal structure. The addition of lithium ions caused the crystal to expand and increased the material’s conduction of the ions. The researchers also learned that the effect works with other types of ions, particularly sodium ions, pointing to potential applications in energy storage.
“The results highlight the potential of quantum materials and emergent physics in the design of ion conductors,” said Shriram Ramanathan, a Purdue University professor of materials engineering who is leading the research. “There is a lot of research now going on to identify solid-state ion conductors for building batteries, for example. We showed that this general family of materials can hold these ions, so we established some general principles for the design of these sorts of solid-state ion conductors. We showed that ions like lithium and sodium can move through this solid material, and this opens up new directions for research.”
Applying a voltage caused the ions to occupy spaces between atoms in the crystal lattice of the material. The effect could represent a more efficient method to store and conduct electricity. Such an effect could lead to new types of batteries and artificial synapses in “neuromorphic,” or brain-inspired, computers. Moreover, the ions remained in place after the current was turned off, a “non-volatile” behavior that might be harnessed for computer memory. Adding lithium ions to the crystal structure also changes the material’s optical properties, suggesting potential applications as coatings for “smart windows” whose light transmission properties are altered when voltage is applied.
The research paper’s lead authors are Purdue University materials engineering postdoctoral research associate Yifei Sun and Michele Kotiuga, a postdoctoral fellow in the Department of Physics and Astronomy at Rutgers University. To develop the doping process, materials engineers collaborated with Vilas Pol, a Purdue associate professor of chemical engineering and materials engineering, and Purdue graduate student Dawgen Lim.
The research findings demonstrated behavior related to the “Mott transition,” a quantum mechanical effect describing how the addition of electrons can change the conducting behavior of a material.
“As we add more electrons to the system the material becomes less and less conducting, which makes it a very interesting system to study, and this effect can only be explained through quantum mechanics,” Ramanathan said.
Kotiuga’s contribution to the work was to study the electronic properties of lithium-doped samarium nickelate as well as the changes to the crystal structure after doping.
“My calculations show that undoped samarium nickelate is a narrow-gapped semiconductor, meaning that even though it is not metallic, electrons can be excited into a conducting state without too much trouble,” said Kotiuga. “As lithium is added to samarium nickelate the lithium ion will bind to an oxygen and an electron localizes on a nearby nickel-oxygen octahedron, and when an electron has localized on every nickel-oxygen octahedron the material is converted into an insulator. This is a rather counterintuitive result: the added electrons to the system make the material more insulating.”
The material’s crystal structure was characterized using the X-ray Science Division x-ray beamlines 12-ID-D, 20-ID-C, and 33-ID-C of the APS at Argonne National Laboratory, and the 23-ID-2 beamline of National Synchrotron Light Source II at Brookhaven National Laboratory. Both are Office of Science user facilities.
The researchers had been working on the paper for about two years and plan to further explore the material’s quantum behavior and potential applications in brain-inspired computing.
See: Yifei Sun1, Michele Kotiuga2*, Dawgen Lim1, Badri Narayanan3, Mathew Cherukara3, Zhen Zhang1, Yongqi Dong3, Ronghui Kou3, Cheng-Jun Sun3, Qiyang Lu4, Iradwikanari Waluyo5, Adrian Hunt5, Hidekazu Tanaka6, Azusa N. Hattori6, Sampath Gamage7, Yohannes Abate7, Vilas G. Pol1, Hua Zhou3, Subramanian K.R.S. Sankaranarayanan3, Bilge Yildize4, Karin M. Rabe2**, and Shriram Ramanathan1, “Strongly correlated perovskite lithium ion shuttles,” Proc. Nat. Acad. Sci. USA, 201805029, published ahead of print August 13, 2018.
Author affiliations: 1Purdue University, 2Rutgers, The State University of New Jersey, 3Argonne National Laboratory, 4Massachusetts Institute of Technology, 5Brookhaven National Laboratory, 6Osaka University, 7University of Georgia
Correspondence: *mkotiuga@physics.rutgers.edu, ** kmrabe@physics.rutgers.edu
We acknowledge National Science Foundation (NSF) Grant 1609898 and Air Force Office of Scientific Research Grant FA9550-16-1-0159 for support. M.K. and K.M.R. acknowledge support from Office of Naval Research Grant N00014-17-1-2770. D.L. and V.G.P. acknowledge support from Office of Naval Research Grant N00014-18-1-2397. This research used resources of the Argonne Leadership Computing Facility, which is a U.S. Department of Energy (DOE) Office of Science User Facility supported under Contract DE-AC02-06CH11357. Use of the Center for Nanoscale Materials, an Office of Science User Facility, was supported by the U.S. DOE Office of Science-Basic Energy Sciences, under Contract DE-AC02-06CH11357. This research used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the U.S. DOE under Contract DE-AC02-05CH11231. Z.Z. acknowledges support from Army Research Office Grant W911NF-16-1-0042. This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. APS Sector 20 operations are supported by the U.S. DOE and the Canadian Light Source. Q.L. and B.Y. acknowledge funding support from the Massachusetts Institute of Technology Materials Research Science and Engineering Center (MRSEC) through the MRSEC Program of the NSF under Award DMR-1419807. This research used resources of the National Synchrotron Light Source II, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract DE-SC0012704. H.T. and A.H. acknowledge Japan Society for the Promotion of Science KAKENHI Grant 15KK0236. S.G. acknowledges support provided by National Science Foundation Grant 1553251. The work of Y.A. was supported by the Air Force Office of Scientific Research Grant FA9559-16-1-0172.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
