The original University of North Carolina School of Medicine press release by Mark Derewicz can be read here.
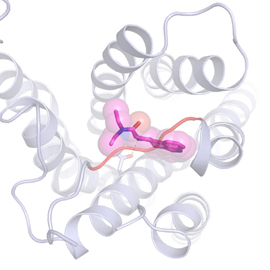
For the first time, scientists at the University of North Carolina (UNC) and the University of North Carolina School of Medicine, together with colleagues at Stanford University, the Stanford University School of Medicine, and the University of California, San Francisco, carrying out research at the U.S. Department of Energy’s Advanced Photon Source (APS), have discovered precisely what the drug lysergic acid diethylamide (LSD) looks like in its active state when attached to a human serotonin receptor of a brain cell, and their first-ever crystal structure revealed a major clue for why the psychoactive effects of LSD last so long. Solving the structure of LSD could help drug developers design better psychiatric drugs with fewer side effects.
The research was published in the journal Cell.
“There are different levels of understanding for how drugs like LSD work,” said Bryan L. Roth, the Michael Hooker Distinguished Professor of Protein Therapeutics and Translational Proteomics in the UNC School of Medicine. “The most fundamental level is to find out how the drug binds to a receptor on a cell. The only way to do that is to solve the structure. And to do that, you need x-ray crystallography, the gold standard.”
That is what Roth’s lab accomplished – essentially “freezing” LSD attached to a receptor so his team could capture crystallography images at the National Institute of General Medical Sciences and National Cancer Institute GM/CA-XSD) x-ray beamline at the Argonne National Laboratory APS (the APS is an Office of Science user facility). As it turns out, when LSD latches onto a brain cell’s serotonin receptor, the LSD molecule is locked into place because part of the receptor folds over the drug molecule, like a lid. And then it stays put.
“We think this lid is likely why the effects of LSD can last so long,” said Roth. “LSD takes a long time to get onto the receptor, and then once it’s on, it doesn’t come off. And the reason is this lid.”
Eventually, though, an acid trip ends. Some LSD molecules pop off their receptors as the lid moves around. Also, brain cells eventually respond to this strange molecule by sucking the receptor into the cell, where it – along with the LSD – is degraded or disassembled for recycling.
Postdoctoral researchers and article co-first authors Daniel Wacker and Sheng Wang led the experiments to crystallize LSD bound to a serotonin receptor and discover why it stays bound so long. “Serotonin, obviously, hits this receptor on brain cells,” Wacker said. “But our experiments show that serotonin does not interact with this lid in the same way LSD does.”
Although other labs have reported that LSD “washes” out of the brain’s fluid within four hours, such experiments could not determine what was happening on or inside brain cells. Roth’s lab has shown for the first time that LSD is not washed out of the serotonin receptors located within the membrane of brain cells in a few hours.
How this popular drug causes such powerful effects has remained a mystery ever since Swiss scientist Albert Hofmann first accidently synthesized and dosed LSD to report its effects in 1938. Now, because of the work of Roth’s lab, scientists can begin to parse how the drug sparks such a dramatic reaction in the brain.
For two decades, Roth’s lab – first at Case Western Research University and then upon his arrival at UNC in 2005 – had been trying to crystalize LSD attached to its receptor through a series of tedious and unsuccessful experiments. Others, too, have been trying. Without crystals, no one would be able to see what LSD bound to a receptor would look like.
“To get crystals of a known compound bound to its receptor is incredibly difficult,” said Roth, who is also director of the National Institute of Mental Health's Psychoactive Drug Screening Program housed at UNC. “In some cases, it’s nearly impossible.”
For the past several years under Roth’s guidance, the task fell to Wacker, who was the first scientist to determine the crystal structure of a serotonin receptor. That was nearly four years ago as a graduate student in the lab of Ray Stevens formerly at the Scripps Institute.
There are a few reasons why crystallizing LSD bound to a receptor is difficult. The first is lack of material; the receptors need to be produced in the lab using a number of tricks such as generating a virus that than infects cells and generates the receptor. Second, the receptors are incredibly flexible, even when compounds such as LSD are bound to them; the receptors do not want to sit still. Third, unlike, say, a molecule of water, a serotonin receptor is highly complex and composed of thousands of atoms.
Wacker explained: “We need a lot of receptors to generate an image because of their small size – much smaller than the wavelength of visible light. Instead we use x-rays, but for that to work we need all of these receptors to sit perfectly still, and they all need to sit still in the same exact way, and that’s what happens in crystals. So, even if you create a lot of serotonin receptors and attempt to crystallize them, one receptor might twitch in one direction, another receptor might twitch in another direction, a third receptor might not be bound to the LSD, and a fourth receptor might have a lid that moves a little more than the other receptors. So, we need to dissolve all these receptors in water and then slowly take away the water. The temperature needs to be just right. And then we need to employ all kinds of experimental tricks to continue to draw out the water and convince the molecules to sit still so that they will want to crystallize.”
It’s sort of like letting soup sit out overnight, Wacker said. You’ll notice salt crystals at the bottom. That’s because the salt in the soup is dissolved in water, but then as water slowly evaporated over time, salt molecules latch onto each other to stay stable. The result: crystals.
But serotonin receptors are not soup. Getting serotonin-LSD crystals took Wacker and colleagues two years, but once they got crystals, the serotonin receptors with LSD were packed tightly together. And that allowed them to shoot x-rays at the receptors, which allowed them to create images of atomic resolution.
Then UNC postdoctoral researcher and article co-first author John McCorvy discovered that the lid was the key to LSD being bound to its serotonin receptor. McCorvy and colleagues created mutant receptors with floppier lids, and they found that LSD bound more quickly and also detached from the receptor more easily. They also noticed that the shorter binding times led to different signaling patterns inside cells. These different patterns likely means that the effects of LSD would have been different than the typical effects with the lid tightly secured.
Co-author Ron Dror and his team at Stanford University used computer simulations to confirm that this is what might happen when LSD engages its receptor protein in a human brain.
“There is a headache drug that binds to the same receptor as LSD,” Dror said. “The two drugs bind in the same receptor pocket, but the shape of that binding pocket is different when one drug or the other is bound. We used computer simulations to help explain why the two drugs favor different binding pocket shapes.
Another aspect of this computational work focused on the fact that the receptor site is not static—the receptor and the drug are both highly dynamic. “They wiggle around all the time, Dror said. “It has long been observed that LSD trips are long. The simulations helped explain why the receptor holds onto LSD for so long despite the fact that they have such a dynamic connection.”
Roth said, “We do not advocate using LSD; it is potentially very dangerous. Now that we’ve solved the structure of LSD bound to a receptor, we are learning what makes it so potent.”
Wacker added, “I think it’s important for the pharmaceutical industry to understand that if you modify just one tiny aspect of any compound, you might affect the way it sits in the receptor. And as a result, you might affect how the compound works.”
See: Daniel Wacker1*, Sheng Wang1, John D. McCorvy1, Robin M. Betz2,3, A.J. Venkatakrishnan2,3, Anat Levit4, Katherine Lansu1, Zachary L. Schools1, Tao Che1, David E. Nichols5, Brian K. Shoichet4, Ron O. Dror2,3**, and Bryan L. Roth1,7***, “Crystal Structure of an LSD-Bound Human Serotonin Receptor,” Cell 168, 377 (January 26, 2017). DOI: 10.1016/j.cell.2016.12.033
Author affiliations: 1University of North Carolina at Chapel Hill School of Medicine, 2Stanford University, 3Stanford University School of Medicine, 4University of California, San Francisco, 5University of North Carolina at Chapel Hill
Correspondence: *dwacker@email.unc.edu, **ron.dror@stanford.edu, ***bryan_roth@med.unc.edu
This work was supported by National Institutes of Health (NIH) grants RO1MH61887 and U19MH82441, the National Institute of Mental Health Psychoactive Drug Screening Program Contract (all to B.L.R.), GM59957 (to B.K.S.), a Terman Faculty Fellowship (R.O.D.), and the Michael Hooker Distinguished Chair of Pharmacology (B.L.R.). The CRISPR-generated knockout HEK293 cells were a generous gift from A. Inoue (Tohoku University). We also gratefully acknowledge J. Sondek and S. Endo-Streeter for providing independent structure quality control analysis; M.J. Miley and the UNC macromolecular crystallization core for advice and use of their equipment for crystal harvesting and transport, which is supported by the National Cancer Institute under award number P30CA016086; J. Smith, R. Fischetti, and the staff of GM/CA-XSD, which has been funded with federal funds from the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
See also: "How LSD Binds to the Brain, and Why Trips Last So Long," by Douglas Main, Newsweek.com, 1.26.17.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.