Adapted from a University of Texas Health Science Center at San Antonio highlight
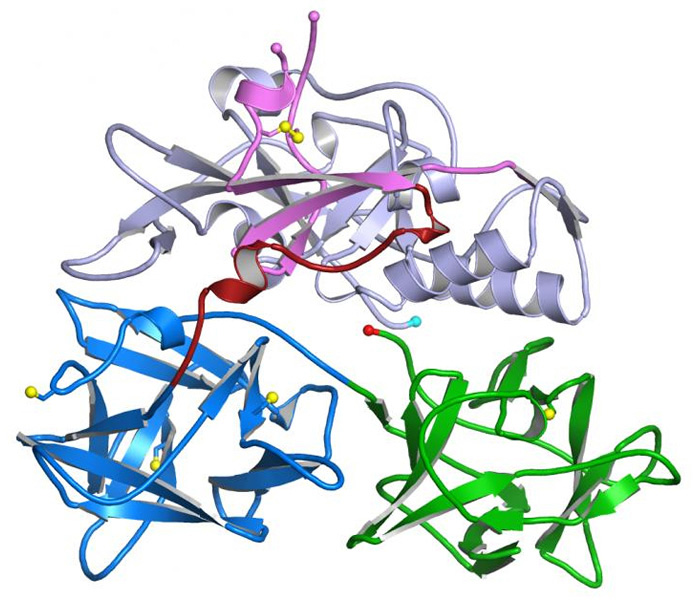
The molecular structure of the cytotoxin from Mycoplasma pneumoniae, a widespread, highly contagious bacterium that infects the lungs, has been determined by a research team using high-brightness x-rays from the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne. The determination of the structure of the protein, called Community Acquired Respiratory Distress Syndrome (CARDS) toxin, could facilitate drug and vaccine development for asthma and other airway diseases.
According to the U.S. Centers for Disease Control and Prevention (CDC), two million new cases of Mycoplasma pneumoniae infections occur each year in the U.S., but the true extent of the health problem is not known and is probably underestimated. It is thought to be responsible for millions of cases of child and adult asthma.
The finding was published in the Proceedings of the National Academy of Sciences. “We know a lot about how the toxin works, but we did not have its three-dimensional structure,” said corresponding author Joel B. Baseman of The University of Texas (UT) Health Science Center at San Antonio. "The structure shows us the molecular architecture of the protein, which permits the rational design of effective drugs and vaccines to neutralize the injurious effects of CARDS toxin.”
The structure was the result of x-ray diffraction data obtained at the Northeastern Collaborative Access Team 24-ID-E x-ray beamline at the APS by a team of researchers from UT Health Science Center, Old Dominion University, St. Mary’s University, and the U.S. Department of Veterans Affairs.
“The importance of the structure is that it gives us a detailed picture of the protein machine that causes the damage in the lungs linked to asthma and adult respiratory distress syndrome (ARDS),” said lead author P. John Hart of the UT Health Science Center Center and Department of Veterans Affairs, South Texas Veterans Health Care System.
Mycoplasmas are the smallest of all bacteria and have proven very difficult to study. Baseman's laboratory discovered the CARDS Toxin in 2006, a finding that was called, at the time, one of the most important in the field since the discovery of the classical toxins of cholera, diphtheria and pertussis. The latter, pertussis toxin, is the agent that causes whooping cough.
The CARDS toxin structure is unique, said Hart. “In comparison to cholera and pertussis toxins, which have similar action and molecular architectures, the overall organization of CARDS toxin is completely different.”
Mycoplasma pneumoniae is spread by sneezing, coughing, talking, and touching hands to the nose. This bacterium infects and colonizes the lungs and secretes the CARDS toxin, resulting in extensive airway injury accompanied by fever, excessive mucus secretion, wheezing and hyper-inflammation. Blocking the toxin could prove to be a major advance in the prevention and care of a wide range of acute and chronic airway diseases, Baseman said.
See: Argentina Becker1, T. R. Kannan1, Alexander B. Taylor1, Olga N. Pakhomova1,2, Yanfeng Zhang1, Sudha R. Somarajan1, Ahmad Galaleldeen1,3, Stephen P. Holloway1, Joel B. Baseman1*, and P. John Hart1,4**, “Structure of CARDS toxin, a unique ADP-ribosylating and vacuolating cytotoxin from Mycoplasma pneumonia,” Proc. Natl. Acad. Sci. 112(16), 5165 (April 21, 2015). DOI: 10.1073/pnas.1420308112
Author affiliations: 1University of Texas Health Science Center at San Antonio, 2Old Dominion University, 3St. Mary’s University, 4U.S. Department of Veterans Affairs
Correspondence: *baseman@uthscsa.edu, **pjh@biochem.uthscsa.edu
J.B.B., P.J.H., and coauthors are supported by National Institutes of Health Grant U19 AI070412. J.B.B. is also supported by the Kleberg Foundation. P.J.H. is also supported by R. A. Welch Foundation Grant AQ-1399. The X-ray Crystallography Core Laboratory is supported by the Office of the Vice President for Research and by P30 CA054174 to Cancer Therapy and Research Center, UTHSCSA.Support for the Northeastern Collaborative Access Team sector at the APS is provided by National Institutes of Health Grant P41 GM103403 and U.S. Department of Energy Grant DE-AC02–06CH11357. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.