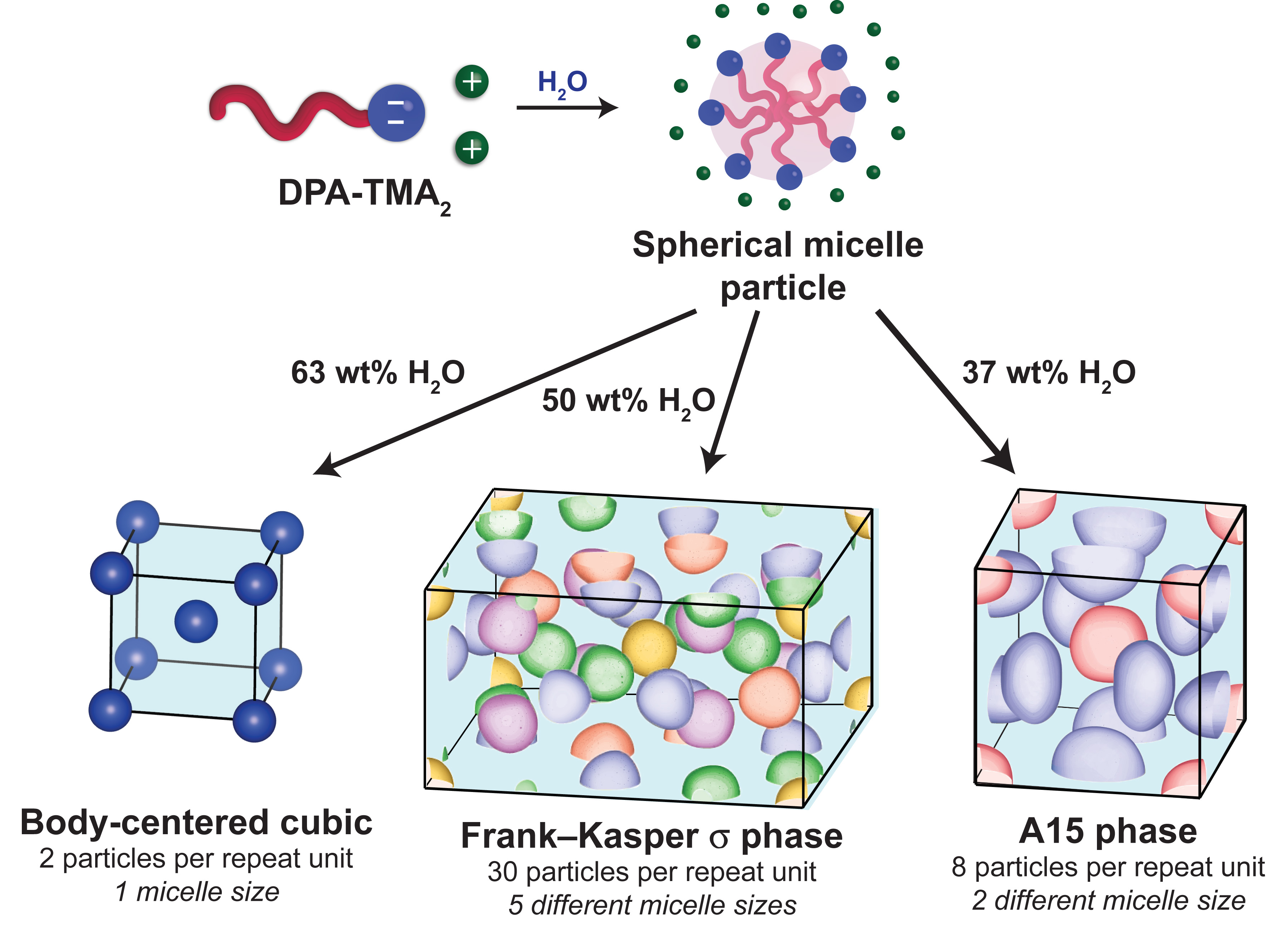
Soft particles, including colloidal particles and various types of polymers and liquid crystals, can self-assemble into useful materials that serve as catalysts, microscale materials for manipulating light, and therapeutic drug delivery vehicles, amongst myriad other applications. Learning how to manipulate their microscopic arrangements, which in turn influences their macroscopic properties, requires a fundamental understanding of the interplay between the structures and symmetries of the particles and how they interact. In water, surfactant molecules—key components of soaps—self-assemble into soft spherical particles that have long been thought to assume the same close-packed configurations as hard spheres, such as stacks of oranges at a grocery store. However, a new study by a team of researchers from the University of Minnesota and the University of Wisconsin–Madison demonstrates that the situation can be far more complex. Utilizing information collected at the U.S. Department of Energy’s Advanced Photon Source (APS), the researchers found that mixing a particular surfactant known as bis(tetramethylammonium) decylphosphonate (DPA-TMA2) with precise amounts of water drives its self-assembly into intricate structured solids involving packings of spheres of up to five different sizes that interact through inherent electrical charges. The findings suggest that other charged spherical particles might also self-assemble in water into unusual configurations, all of which could have potentially useful applications.
The researchers started by synthesizing pure samples of DPA-TMA2. Mixing defined ratios of this surfactant and ultrapure water gives rise to soft solids in which the surfactant molecules spontaneously form spherical particles called micelles that pack into periodic 3D structures. The researchers then used the X-ray Science Division 12-ID-B and the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) 5-ID-D beamlines at the Argonne National Laboratory APS to carry out synchrotron small-angle x-ray scattering experiments to investigate the exact packing arrangements of the micelles. (The APS is an Office of Science user facility.)
Their findings revealed a zoo of unexpected structures that depend on the relative amounts of water and surfactant that are mixed. When small amounts of DPA-TMA2 are mixed with a large amount of water, the micelles do not pack into any organized structure and instead form freely flowing fluids. Decreasing the amount of water mixed with surfactant leads to a body-centered cubic packing of micelles, in which the repeating unit is a cube containing a central micelle that is surrounded by eight nearest neighbors situated at the cube corners. At medium water concentrations, SAXS analyses surprisingly showed that the surfactant micelles organize into a tetrahedral close packing with a giant, repeating unit cell containing 30 deformed spherical micelles. This arrangement, they say, fits the pattern of a Frank-Kasper sigma phase, a crystal structure taken on by certain heavy metals (e.g., uranium and tantalum) and intermetallic alloys (e.g., FeCr). A curious feature of this unusual structure is that its repeating pattern contains micelles of five discrete sizes, rather than a single uniform micelle as anticipated by conventional wisdom. The surfactant micelles adopt another periodic crystal structure known as the A15 phase at even lower water concentrations. This micellar A15 structure is also an analog of a known intermetallic structure.
Utilizing computer simulations, the researchers demonstrated that the formation of these complex structures arises from a competition between the spherical symmetry of the micelle particles and the overall symmetry of the packing that they adopt. Packing soft micelles into a regular structure distorts their shape through interactions between micelles that are mediated by clouds of charged particles, or ions, that surround each sphere. However, the ion cloud around each sphere prefers to maintain a spherical shape. To accommodate these competing effects, the particles spontaneously exchange surfactant chains to reconfigure into multiple spheres of different sizes that pack into more complex crystal structures with large unit cells.
The authors note that the structurally periodic arrangements of these soft surfactant micelles are direct analogs of structures of hard metal alloys. Since the properties that appear to be responsible for these surprising crystal structures are present in other types of spherical, ionic particles in solution, the researchers suggest that further investigations of these and related materials could drive the discovery of other soft particle packings with unusual properties that could offer novel ways to manipulate light and other forms of energy. — Christen Brownlee
See: Sung A. Kim1, Kyeong-Jun Jeong2, Arun Yethiraj2, and Mahesh K. Mahanthappa1*, “Low-symmetry sphere packings of simple surfactant micelles induced by ionic sphericity,” Proc. Natl. Acad. Sci. USA 114(16), 4072 (April 18, 2017). DOI: 10.1073/pnas.1701608114
Author affiliations: 1University of Minnesota, 2University of Wisconsin–Madison
Correspondence: *maheshkm@UMN.edu
This work was supported by the U.S. Department of Energy (DOE)-Basic Energy Sciences, Contract No. DE-SC0010328. DND-CAT is supported by E. I. DuPont de Nemours & Co., The Dow Chemical Company, and Northwestern University. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.