The original Carnegie Institute of Science press release can be read here.
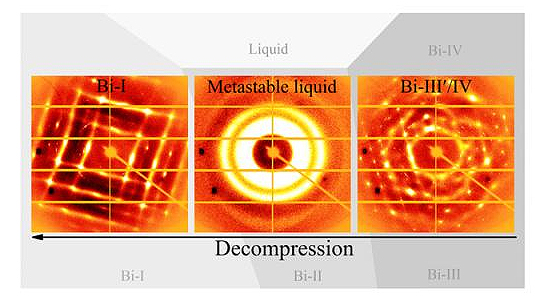
Phase transitions surround us—for instance, liquid water changes to ice when frozen and to steam when boiled. Now, researchers at the Carnegie Institution for Science utilized the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne National Laboratory in their discovery of a new phenomenon of so-called metastability in a liquid phase. A metastable liquid is not quite stable. This state is common in supercooled liquids, which are liquids that cool below the freezing point without turning into a solid or a crystal. These scientists report the first experimental evidence of creating a metastable liquid directly by the opposite approach: melting a high-pressure solid crystal of the metal bismuth via a decompression process below its melting point. The results, reported in the January 23, 2017, issue of Nature Communications, could be important for developing new materials and for understanding the dynamics of planetary interiors, such as earthquakes, because a metastable liquid could act as a lubricant strongly affecting the dynamics of the Earth's interior.
“Phase transitions come in two basic ‘flavors,’” said Carnegie co-author Guoyin Shen, Director of the High-Pressure Collaborative Access Team (HP-CAT) at the APS (the APS is an Office of Science user facility). “In one type, the chemical bonds do not break as the material goes from one phase to another. But they do alter in orientation and length in an orderly manner. The other, called reconstructive phase transition, is more chaotic, but the most prevalent in nature and the focus of this study. In these transitions, parts of the chemical bonds are broken and the structure changes significantly when it enters a new phase,” Shen said,
Pressure can be utilized to change the phase of a material, in addition to heating and cooling. The scientists in this study put a form of crystalline bismuth in a pressure-inducing diamond anvil cell at the HP-CAT x-ray beamline at the APS, and subjected it to pressures and decompression ranging from 32,000 times atmospheric pressure (3.2 GPa) to 12,000 atmospheres (1.2 GPa) at a temperature of 420° F (489 K). Under decompression only, at about 23,000 atmospheres, bismuth melted into a liquid. Then at 12,000 atmospheres it recrystallized.
“The richness in crystalline structure of bismuth is particularly useful for witnessing changes in the structure of a material,” said Chuanlong Lin, lead author of the Nature Communications article.
The researchers imaged the changes using a technique called x-ray diffraction, which uses much higher energy x-rays than those used for medical imaging and can therefore discern structure at the atomic level. They conducted five different compression/decompression rounds of experiments at HP-CAT beamlines 16-ID-B and 16-BM-D.
“The bismuth displayed a metastable liquid in the process of solid-solid phase transitions under decompression at about 23,000 to 15,000 atmospheres,” Lin said.
The scientists also found that the metastable state can endure for hours below the melting point under static conditions. Interestingly, the metastable liquid produced by decompression occurred in a pressure-temperature range that is similar to where supercooled bismuth is produced.
“Because reconstructive phase transitions are the most fundamental type, this research provides a brand new way for understanding how different materials change,” Shen said. “It's possible that other materials could display a similar metastable liquid when they undergo reconstructive transitions and that this phenomenon is more prevalent than we thought. The results will no doubt lead to countless surprises in both materials science and planetary science in the coming years.”
See: Chuanlong Lin, Jesse S. Smith, Stanislav V. Sinogeikin, Yoshio Kono, Changyong Park, Curtis Kenney-Benson, and Guoyin Shen*, “A metastable liquid melted from a crystalline solid under decompression,” Nat. Commun. 8, 14260 (2016). DOI: 10.1038/ncomms14260
Author affiliation: Carnegie Institution of Washington
Correspondence: *gshen@ciw.edu
This research was supported by U.S. Department of Energy (DOE)-Basic Energy Sciences (BES), Division of Materials Sciences and Engineering under Award DE-FG02- 99ER45775, and DOE-National Nuclear Security Administration (NNSA), Stewardship Science Academic Programs under Award DE-NA0001974. HP-CAT operations are supported by DOE-NNSA under Award No. DE-NA0001974 and DOE-BES under Award No. DE-FG02-99ER45775, with partial instrumentation funding by the National Science Foundation (NSF). Use of the COMPRES-GSECARS gas-loading system was supported by COMPRES under NSF Cooperative Agreement EAR 11–57758 and by GSECARS through NSF grant EAR-1128799 and DOE grant DE-FG02-94ER14466. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.