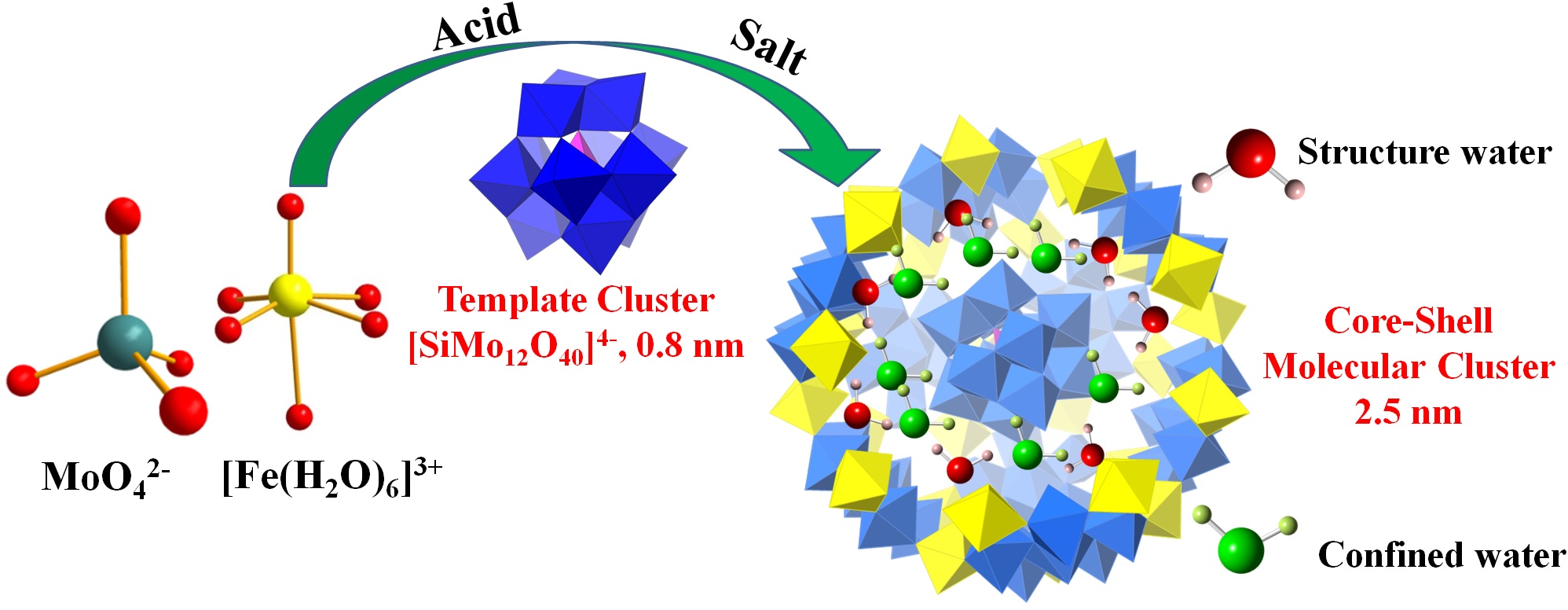
Polyoxometalates (POMs) are giant molecules, a few nanometers across, with various morphologies and compositions. Created in multi-hour chemical syntheses from small molecular reactants, they can have tunable redox and surface properties that make them useful as catalysts, as well as electric and magnetic properties with possible applications in nanomaterials. Until now, however, the formation mechanism of POMS has been poorly understood. By combining small-angle x-ray scattering at the U.S. Department of Energy’s Advanced Photon Source (APS) and neutron scattering techniques, a team of researchers has now provided a clearer picture of the structure of these complex species and has also inferred a formation process that goes through three distinct stages, leading to crystallization. The findings should make it easier to devise synthesis routes for creating POMs with desirable customized characteristics.
The researchers from Oak Ridge National Laboratory (ORNL), the University of Tennessee, and Argonne National Laboratory focused their attention on POMs with a core-shell structure. The shells of such POMs consist of transition metals linked into a framework connected together by oxo ligands. When such a shell encloses a core, typically also consisting of metal oxides, it results in what is known as a Keggin structure. Synthesis of POMs follows a standard route, in which the ionic core structure, already made, is placed in an acidic solution of transition metal oxides or ions. The core-shell structure precipitates out as a salt in a process that can occupy 24 hours. Mass spectrometry of samples from such reactions, taken at various times, have given some clues as to the nature of intermediate structures involved in the synthesis, but the sample extraction and preparation steps necessary for such studies cast doubt on whether the structures found are truly building blocks of the finished POM.
The researchers chose to study a representative POM consisting of a silicon-molybdenum oxide core within an iron-molybdenum oxide shell (Fig. 1). The shell contained 72 molybdenum atoms and 30 iron atoms, and measured 2.5 nm in diameter. They used small-angle x-ray scattering (SAXS) at X-ray Science Division x-ray beamlines 12-ID-B and 12-ID-C at the APS, an Office of Science user facility at Argonne, along with elastic and inelastic neutron scattering conducted at the U.S. Department of Energy’s (DOE’s) Spallation Neutron Source at ORNL, to follow the synthesis reaction in situ.
The SAXS measurements, on reactant solutions in quartz capillaries, provided a spatial resolution up to 40 nm and time resolution better than 0.1 s. First, the team studied POMs that had crystallized in their final state in order to determine the location of the core within the shell — not an easy task since the orientation of each core within its shell is random. By using an iterative modeling procedure that computed SAXS signals in which the cores had all possible alignments and were displaced by small distances from the centers of the shells, the researchers found a best fit to the result when the cores were strictly central in the structures.
Time-resolved SAXS studies made it possible to track the formation of the POMs over many hours. About 2.5 hours after the reaction started, signs of shell formation began to appear. (When the reactants were mixed together without the cores, no shell structures appeared even after 24 hours). The concentration of shells gradually increased, but at the 19-hour mark, structure factor analysis indicated that they were systematically staying away from each other. Only after that time, when the shell concentration had increased to a presumably critical value, did the structures begin to collect together in crystalline form. Measurements of some samples about two weeks after synthesis showed the same structure, indicating the long-term stability of the POMs in aqueous solutions.
While the SAXS experiments revealed how the metal ions came together in the structures, the neutron scattering studies focused on the presence and location of water molecules. Two significant results emerged. Quasi-elastic scattering indicated the presence of water molecules that were mobile, but more sluggish than those in plain water. The researchers concluded that there are water molecules confined between the core and the shell, but able to move within this restricted space. In addition, inelastic peaks in the neutron scattering spectra pointed to the presence of fixed water molecules, which the team inferred are incorporated into molecular bridges that link core and shell. Both types of water molecule, they say, contribute to the structural stability and longevity of the POMs.
This new insight into the mechanism by which POMs form should make it easier to devise structures with different compositions and symmetries, opening up possibilities for applications such as novel chemical catalysts or single-molecule magnets for digital memories.
— David Lindley
See: Panchao Yin1*, Bin Wu2, Eugene Mamontov1, Luke L. Daemen1, Yongqiang Cheng1, Tao Li3, Soenke Seifert3, Kunlun Hong1, Peter V. Bonnesen1, Jong Kahk Keum1, and Anibal J. Ramirez-Cuesta1, “X‑ray and Neutron Scattering Study of the Formation of Core−Shell-Type Polyoxometalates,” J. Am. Chem. Soc. 138, 2638 (2016). DOI: 10.1021/jacs.5b11465
Author affiliations: 1Oak Ridge National Laboratory, 2University of Tennessee, 3Argonne National Laboratory
Correspondence: *yinp@ornl.gov
P.Y. is grateful to the support of Clifford G. Shull Fellowship from the Neutron Sciences Directorate of ORNL. The research performed in BL-2 (BASIS) and BL- 16B (VISION) at ORNL’s Spallation Neutron Source was sponsored by the Scientific User Facilities Division-Basic Energy Sciences, U.S. DOE. The sample preparation and initial SAXS study in the x-ray lab were conducted at the ORNL Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility. Oak Ridge National Laboratory is supported by the Office of Science of the U.S. DOE under Contract No. DE-AC05- 00OR22725. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02- 06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.