The original UT Southwestern Medical Center press release can be read here.
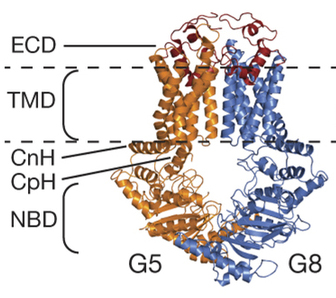
Using x-ray crystallography at the U.S. Department of Energy’s Advanced Photon Source (APS), UT Southwestern Medical Center and Texas Tech University Health Sciences Center researchers have determined the three-dimensional (3-D) atomic structure of a human sterol transporter that helps maintain cholesterol balance.
Cholesterol is an essential component of cell membranes. Two ATP binding cassette (ABC) half transporters, ABCG5 and ABCG8, form a complex that transports sterols across membranes.
The researchers solved the structure of the ABCG5/ABCG8 heterodimer (G5G8), which mediates excretion of neutral sterols in the liver and intestines, by collecting x-ray diffraction data at the Structural Biology Center Collaborative Access Team (SBC-CAT) 19-ID-D and National Institute of General Medical Sciences and National Cancer Institute (GM/CA-XSD) 23-ID-D x-ray beamlines at the APS. (The APS is an Office of Science user facility at Argonne.)
“Determining the structure of this protein complex helps us to understand the mechanism by which the two component proteins work together to clear sterols from the body,” said Daniel Rosenbaum, Assistant Professor of Biophysics and Biochemistry at UT Southwestern and a corresponding author of the study, which was published Nature. “This knowledge, in turn, could lead to finding highly targeted therapies to treat or prevent diseases related to sterol imbalance.” Helen Hobbs, a Howard Hughes Medical Institute Investigator and Director of the Eugene McDermott Center for Human Growth and Development as well as a recent recipient of the $3 million Breakthrough Prize in Life Sciences for her work on cholesterol genetics, is the study’s other corresponding author.
Specifically, the ABCG5/ABCG8 complex is involved in excretion of sterols from the liver and the intestines. Disruption by mutations in either protein can lead to the disorder sitosterolemia. Patients with sitosterolemia have elevated levels of cholesterol and other sterols in their tissues and blood, which can cause heart attacks at an early age, Rosenbaum said.
Under normal conditions, animals maintain sterol balance by limiting dietary sterol uptake from the gut and promoting sterol secretion from the liver into the bile, added Rosenbaum.
Lead author Jyh-Yeuan “Eric” Lee, a research scientist in the McDermott Center, said humans have 48 ABC transporters that are classified into seven subfamilies, called A to G.
“The structure we deduced for the ABCG5/ABCG8 complex represents a new type of ABC transporter architecture, and is the first mammalian ABC transporter to be crystallized in a lipid bilayer and solved to atomic resolution,” Lee said.
In addition, Rosenbaum said, the study data could provide insight into the structural elucidation of other ABC transporters, most of which remain uncharacterized.
See: Jyh-Yeuan Lee1, Lisa N. Kinch1, Dominika M. Borek1, Jin Wang1, Junmei Wang1, Ina L. Urbatsch2, Xiao-Song Xie1, Nikolai V. Grishin1, Jonathan C. Cohen1, Zbyszek Otwinowski1, Helen H. Hobbs1*, and Daniel M. Rosenbaum1**, “Crystal structure of the human sterol transporter ABCG5/ABCG8,” Nature 533, 561 (26 May 2016). DOI: 10.1038/nature17666
Author affiliations: 1University of Texas Southwestern Medical Center, 2Texas Tech University Health Sciences Center
Correspondence: *Helen.Hobbs@UTSouthwestern.edu, **Dan.Rosenbaum@UTSouthwestern.edu
This project was supported by grants from the American Heart Association South Central Affiliate (0825285F; J.-Y.L.), the American Heart Association Texas Affiliate Beginning Grant-in-Aid (0463130Y; I.L.U.), the Welch Foundation (I-1770; D.M.R.), the Packard Foundation (D.M.R.), the Howard Hughes Medical Institute (H.H.H., N.V.G.), and the National Institutes of Health (HL72304 and P01-HL20948 (H.H.H., J.C.C., X.-S.X.), GM094575 (N.V.G.), GM053163 and GM117080 (Z.O.), and GM113050 (D.M.R.)). SBC-CAT is operated by UChicago Argonne, LLC, for the U.S. Department of Energy, Office of Biological and Environmental Research under contract DE-AC02-06CH11357. GM/CA-XSD has been funded in whole or in part with Federal funds from the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006).
This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.