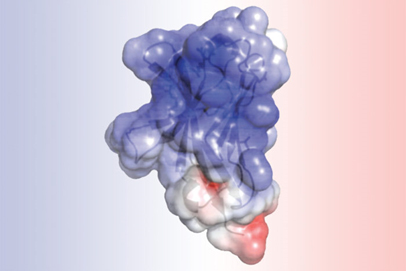
Scientists from the Washington University School of Medicine in St. Louis used the U.S. Department of Energy’s Advanced Photon Source (APS), an Office of Science user facility at Argonne National Laboratory, to detail the structure of a molecule that has been implicated in Alzheimer’s disease. Knowing the shape of the molecule — and how that shape may be disrupted by certain genetic mutations — can help in understanding how Alzheimer’s and other neurodegenerative diseases develop and how to prevent and treat them. The study was published in the December 20, 2016, issue of the journal eLife.
The idea that the molecule TREM2 is involved in cognitive decline — the hallmark of neurodegenerative diseases, including Alzheimer’s — has gained considerable support in recent years. Past studies have demonstrated that certain mutations that alter the structure of TREM2 are associated with an increased risk of developing late-onset Alzheimer’s, frontal temporal dementia, Parkinson’s disease and sporadic amyotrophic lateral sclerosis (ALS). Other TREM2 mutations are linked to Nasu-Hakola disease, a rare inherited condition that causes progressive dementia and death in most patients by age 50.
“We don’t know exactly what dysfunctional TREM2 does to contribute to neurodegeneration, but we know inflammation is the common thread in all these conditions,” said senior author Thomas J. Brett, Assistant Professor of Medicine at Washington University School of Medicine in St. Louis. “Our study looked at these mutations in TREM2 and asked what they do to the structure of the protein itself, and how that might impact its function. If we can understand that, we can begin to look for ways to correct it.”
The analysis of TREM2 structure, obtained at the Structural Biology Center 19-ID-D x-ray beamline at the APS and completed by article first author Daniel L. Kober, a doctoral student in Brett’s lab, revealed that the mutations associated with Alzheimer’s alter the surface of the protein, while those linked to Nasu-Hakola influence the “guts” of the protein. The difference in location could explain the severity of Nasu-Hakula, in which signs of dementia begin in young adulthood. The internal mutations totally disrupt the structure of TREM2, resulting in fewer TREM2 molecules. The surface mutations, in contrast, leave TREM2 intact but likely make it harder for the molecule to connect to proteins or send signals as normal TREM2 molecules would.
TREM2 lies on the surface of immune cells called microglia, which are thought to be important “housekeeping” cells. Via a process called phagocytosis, such cells are responsible for engulfing and cleaning up cellular waste, including the amyloid beta that is known to accumulate in Alzheimer’s disease. If the microglia lack TREM2, or the TREM2 that is present doesn’t function properly, the cellular housekeepers can’t perform their cleanup tasks.
“Exactly what TREM2 does is still an open question,” Brett said. “We know mice without TREM2 have defects in microglia, which are important in maintaining healthy brain biology. Now that we have these structures, we can study how TREM2 works, or doesn’t work, in these neurodegenerative diseases.”
TREM2 also has been implicated in other inflammatory conditions, including chronic obstructive pulmonary disease and stroke, making the structure of TREM2 important for understanding chronic and degenerative diseases throughout the body, Brett added.
See: Daniel L. Kober, Jennifer M. Alexander-Brett, Celeste M. Karch, Carlos Cruchaga, Marco Colonna, Michael J. Holtzman, and Thomas J. Brett*, “Neurodegenerative disease mutations in TREM2 reveal a functional surface and distinct loss-of-function mechanisms,” eLife 5:e20391 (2016). DOI: 10.7554/eLife.20391
Author affiliation: Washington University School of Medicine
Correspondence: *tbrett@wustl.edu
This work was supported in part by funding from National Institutes of Health R01-HL119813 (T.J.B.), Knight Alzheimer’s Disease Research Center pilot grant P50-AG005681-30.1 (T.J.B.), Alzheimer’s Association Research Grant AARG-16-441560 (T.J.B.), K08-HL121168 (J.A.B.), Burroughs-Wellcome Fund Career Award for Medical Scientists (J.A.B.), NIH K01-AG046374 (C.M.K.), R01-AG044546 (C.C.), R01-AG051485 (M.C.), R01- HL120153 (M.J.H.), R01-HL121791 (M.J.H.), T32-GM007067 (D.L.K.), and American Heart Association Pre- doctoral Fellowship PRE22110004 (D.L.K.). The Structural Biology Center at the Advanced Photon Source, Argonne, is operated by UChicago Argonne, LLC, for the U.S. Department of Energy (DOE) Office of Biological and Environmental Research under contract DE-AC02-06CH11357. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.