Researchers from the University of Illinois at Chicago (UIC), the Illinois Institute of Technology (IIT), the Jesse Brown VA Medical Center, and Argonne joined forces and used the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne to understand why superwarfarins, commonly used as rodenticides, are so deadly.
Warfarin, better known as Coumadin®, is an anticoagulant drug used to prevent thrombosis (blood clotting) and thromboembolism in humans, and was also used in the past as rodenticide. Superwarfarins are structurally similar to warfarin, but are much more potent and stay in the body much longer. Even small doses of superwarfarins cause severe bleeding problems in animals and humans. For example, median lethal doses for brodifacoum are between 0.30–0.70 mg/kg of body mass, significantly lower than values reported for warfarin ranging between 10 mg/kg to 300 mg/kg. Due to their high potency and duration of action, superwarfarins may be used as chemical warfare agents. The incidence of superwarfarin poisoning due to accidental or intentional exposure has risen every year and represents a growing public health concern. Accidental poisoning due to ingestion of rodent bait reached over 300,000 cases in the U.S. in 2012. In the same time, the molecular mechanism of action of superwarfarins remained unknown, until recently.
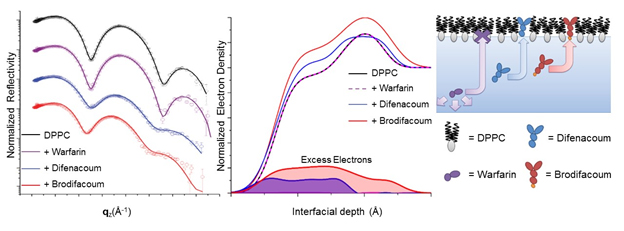
Researchers from UIC and IIT hypothesized that two superwarfarins (brodifacoum and difenacoum) interact with the membranes of host cells. To test this hypothesis, x-ray surface studies of model Langmuir films containing a common phospholipid (dipalmitoylphosphatidylcholine, DPPC) and cholesterol were performed using a liquid surface spectrometer at X-ray Science Division x-ray beamline 9-ID-C at the APS, an Office of Science user facility. Langmuir films were used to mimic one half of the cellular membrane at the air-liquid interface. Various concentrations of warfarin or superwarfarins, brodifacoum and difenacoum, were injected into the subphase, under the Langmuir monolayer, in order to model the interactions of these drugs with the cell membrane. Specular x-ray reflectivity and grazing incidence x-ray diffraction data from beamline 9-ID-C (Fig. 1.) revealed that superwarfarins, but not warfarin, intercalate between the phospholipid molecules in the Langmuir monolayer, suggesting the same mechanism in cellular membranes. These studies on model systems were done at concentrations of superwarfarins and warfarin close to the known lethal doses.
Tissue studies revealed that the presence of an additional bromine atom in the molecular structure of brodifacoum compared to difenacoum resulted in a significant increase in its retention and lethality. Interestingly, the researchers found that the deadlier of the two drugs, brodifacoum, displayed a preferred orientation within the membranes that closely mimicked the orientation of cholesterol, as analyzed by the x-ray reflectivity data.
Consistent with these findings, the researchers were able to demonstrate that superwarfarins induced cell death in neuroblastoma cells. In contrast, these drugs caused no changes in glioma cells that have higher membrane cholesterol. Studies were then redone on glioma cells after cholesterol was depleted from the membrane using cyclodextrins. The glioma cells with depleted levels of cholesterol became susceptible to the superwarfarins. No harmful effects were observed in either cell lines treated with normal warfarin, suggesting that the mechanism of its action is different from superwarfarins.
To see changes on the structural level, surface x-ray studies, also at 9-ID-C, on model membranes with differing levels of cholesterol showed that membranes with as little as 20% cholesterol were resistant to superwarfarins, whereas those without cholesterol were highly susceptible. These findings demonstrate an affinity of superwarfarins to biomembranes and suggest that cellular responses to these agents are regulated by cholesterol content. They demonstrate that the increased potency of superwarfarins is likely due to increased tissue retention within the cellular membranes of specific host cell types. This study highlights a nonspecific, molecular interaction governing the distribution and selectivity of superwarfarins within phospholipid membranes, and suggests that membrane cholesterol can modulate cellular responses to these toxins, and possibly to other deadly drugs.
See: M Natalia Marangoni1, Michael W Martynowycz2,3, Ivan Kuzmenko3, David Braun1, Paul E Polak1, Guy Weinberg1,4, Israel Rubinstein1,4, David Gidalevitz2, Douglas L Feinstein1,4*, “Membrane Cholesterol Modulates Superwarfarin Toxicity,” Biophys. J. 110(8), 1777 (2016). DOI:10.1016/j.bpj.2016.03.004
Author affiliations: 1University of Illinois-Chicago, 2Illinois Institute of Technology, 3Argonne National Laboratory, 4Jesse Brown VA Medical Center
Correspondence: *dlfeins@uic.edu
This work was supported by National Institutes of Health grant 5U01NS083457, Veterans Affairs Merit grant, DARPA grant W911NF-09-1-378. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.