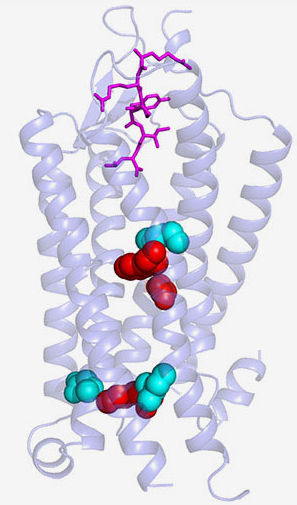
Many hormones and neurotransmitters work by binding to receptors on a cell's exterior surface. This activates receptors causing them to twist, turn and spark chemical reactions inside cells. National Institutes of Health (NIH) scientists used atomic-level images obtained at the U.S. Department of Energy’s Advanced Photon Source (APS), an Office of Science user facility, to show how the neuropeptide hormone neurotensin might activate its receptors. Their description, published in Nature Communications, is the first of its kind for a neuropeptide-binding G protein-coupled receptor (GPCR), a class of receptors involved in a wide range of disorders and the target of many drugs. G protein-coupled receptors are found throughout the body. Knowing how they work should help scientists devise better treatments.
Neurotensin is thought to be involved in Parkinson's disease, schizophrenia, temperature regulation, pain, and cancer cell growth. Previously, the NIH team showed how neurotensin binds to the part of its receptor located on a cell's surface. In this study, they demonstrated how binding changes the structure of the rest of the receptor, which passes through a cell's membrane and into its interior. There, neurotensin receptors activate G proteins, a group of molecules inside cells that controls a series of chemical chain reactions.
For these experiments, scientists utilized x-rays from the National Institute of General Medical Sciences and National Cancer Institute (GM/CA-XSD) x-ray beamline 23-ID-D at the Argonne National Laboratory APS to illuminate crystallized neurotensin receptor molecules. (Initial crystal screening was done at the Stanford Synchrotron Radiation Lightsource [SSRL], beamline 12-2. The SSRL is also an Office of Science user facility.) Making crystals of receptors that activate G proteins is difficult. In most studies, scientists have investigated inactive receptors.
The receptor the team crystallized is very close to the active form found in nature. They may have the first picture of a peptide-binding G protein-coupled receptor just before it engages with the G protein.
To achieve their results, the scientists made multiple genetic modifications to a less active version of the neurotensin receptor they had used before. Experiments performed in test tubes showed that mixing the receptor with neurotensin sparked the G protein reactions for which the scientists were looking.
When the scientists looked at the structure of the new crystals, they discovered how binding of neurotensin to the receptor caused critical parts of the receptor located below a cell's surface to change shape. In particular, they saw that a region in the middle of the receptor dropped like a draw bridge to link the neurotensin binding site to parts of the receptor found inside cells that are important for G protein activation. The scientists concluded that this change may prepare the receptor for activating G proteins.
For years scientists have made educated guesses about how peptide receptors work. These results may finally provide an answer.
The NIH team plans to continue its work in order to fully understand how neurotensin and other G protein-coupled receptors translate messages delivered by neuropeptides into reactions inside cells.
See: Brian E. Krumm, Jim F. White, Priyanka Shah & Reinhard Grisshammer*, “Structural prerequisites for G-protein activation by the neurotensin receptor,” Nat. Commun. 6, 7895 (24 July 2015). DOI: 10.1038/ncomms8895
Author affiliation: National Institutes of Health
Correspondence: *rkgriss@helix.nih.gov
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393). GM/CA-XSD has been funded in whole or in part with Federal funds from the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.