New, highly efficient organic and inorganic light-emitting diodes (OLEDs and LEDs, respectively) and environmental and medical sensors might be made possible by utilizing porous materials known as metal-organic frameworks (MOFs) that combine metallic elements with a diverse range of fluorescent molecules — usually organic molecules — as ligands.
The design and synthesis of rigid, fluorescent materials has been the focus of research aided by the high-brightness x-rays from the U.S. Department of Energy’s Advanced Photon Source (APS)— research that could lead to new applications as well as allowing researchers to fine tune the properties of the materials.
Over the last 50 years or so, electronic devices have been built around semiconductor elements, such as silicon and germanium, or toxic inorganic semiconductors such as gallium arsenide and cadmium sulfide. There are, however, myriads of fluorescent molecules, known as fluorophores, in the realm of organic chemistry that have interesting opto-electronic properties, which could be exploited as novel glowing sensors and OLEDs/LEDs. Even state-of-the-art molecular materials for such applications usually incorporate elements that are rare in Earth’s crust, such as lanthanide metals, whereas the new MOFs herein do not contain such rare elements, hence promoting sustainability and “green chemistry” technologies.
Unfortunately, incorporating such molecules into a working device is difficult because these molecules tend to quench their own fluorescence and so produce only weak light. Additionally, blue light emitters that are both efficient and stable remain the most coveted active device materials, especially for OLEDs.
Researchers from Texas A&M University and the University of North Texas now think they have a solution to these problems by using organic components as the fluorophores of the solid frameworks (bright blue-emitting solid MOFs). The team produced rigid fluorescent MOF materials that respond to external stimuli by glowing strongly with no possibility for the fluorophores to dim through quenching, as occurs in solution or even the solid organic molecule without metal coordination.
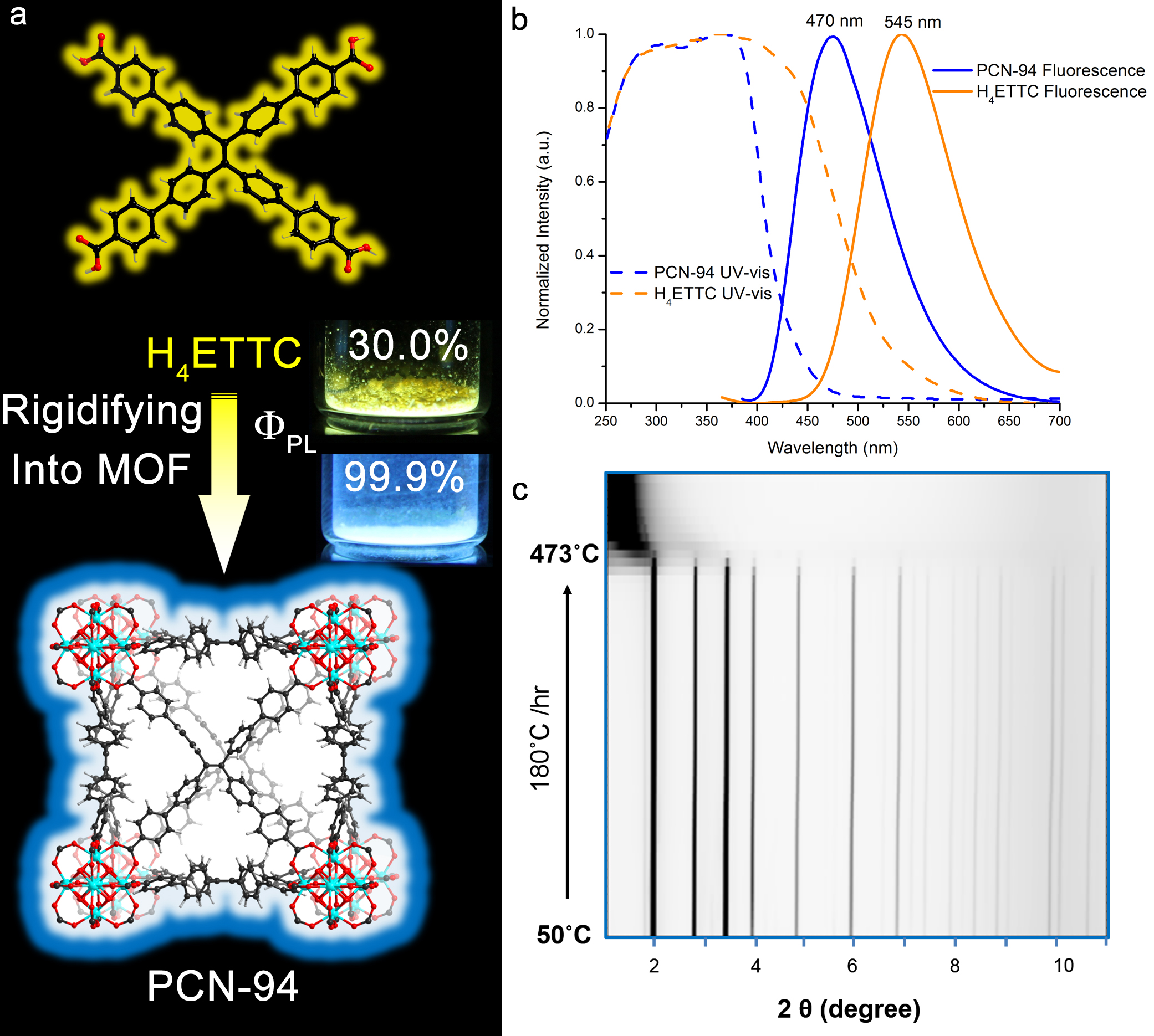
The team has investigated a newly synthesized MOF based on the organic fluorophore, tetraphenylethylene and clusters of the metal zirconium, present in groups of six. The group carried out studies of the MOF, dubbed PCN-94, using powder x-ray diffraction (PXRD) data collected on X-ray Science Division beamline 17-BM at the APS, an Office of Science user facility, to help ascertain its rigid structure that is critical to its fluorescence color and circumvention of quenching.
The new MOF glows with a deep-blue fluorescence at a wavelength of 470 nanometers as peak maximum. The team reports that PCN-94 is highly efficient, which is quantified by the quantum yield (QY) of the material. They found that the QY of PCN-94 is 99.9 ± 0.5% when held under an unreactive atmosphere of argon gas — even at room temperature, which is both remarkable and unprecedented in fluorescent MOFs! Tetraphenylethylene, when coordinated to zirconium metal in the PCN-94, was showing a blue shift with higher efficiency compared to the isolated tetraphenylethylene molecule, which glows a weaker yellow color at a spectral wavelength of 545 nanometers as peak maximum.
Moreover, the fluorescence process is over three times as efficient as the isolated tetraphenylethylene (approximately 100% vs 30% in QY) under complete deaeration conditions, whereas under ambient air the improvement in radiative decay and QY is approximately 2x for the solid MOF vs the free ligand. The team expected efficiency to be greatest at the cryogenic chilling temperature of 110 K (about -163° Celsius) because self-quenching of the emission from the fluorophores is inhibited by the lower temperature, i.e., 110 K vs ambient room temperature (about 293 K or 20° Celsius). Paradoxically, however, they saw the fluorescence lifetime — how long the glow lasts — as well as the relative intensity of the light increase as they warmed the solid MOF from cryogenic to room temperature, whereas the solid organic fluorophores follows the normal behavior with lower emission intensity upon heating from cryogenic toward ambient temperatures. The team reports that this unusual fluorescence behavior is perhaps due to the fact that the fluorophores are “frozen” in their twisted connections between the metals in the framework at all temperatures while heating provides thermal assistance to a secondary excitation mechanism from a higher-lying excited state that feeds the blue-fluorescent excited state. In the free organic molecule, however, the twisting becomes more important as the temperature rises (which again impedes quenching).
The team suggests that their discovery of an efficiency boost and the "shift" of the fluorescent glow of the organic unit from yellow to the strongly sought-after deep-blue could open a new strategy for making OLED/LED and sensor materials that are based on this and other related MOFs that the two complementary research groups are currently designing on the heels of this pioneering work as a backdrop. The field of MOF research has grown rather significantly since the turn of the Millennium and — given that there are dozens of different metals that can be used to build frameworks with thousands of different organic fluorophores —numerous possibilities exist toward building MOFs that fluoresce across the entire visible spectrum with similarly-remarkable efficiency as that of PCN-94 for a wide range of molecular electronic device and sensor applications.— David Bradley
See: Zhangwen Wei1, Zhi-Yuan Gu1, Ravi K. Arvapally2, Ying-Pin Chen1,2, Roy N. McDougald, Jr.2, Joshua F. Ivy2, Andrey A. Yakovenko1, Dawei Feng1, Mohammad A. Omary2,** and Hong-Cai Zhou1,2*, “Rigidifying Fluorescent Linkers by Metal−Organic Framework Formation for Fluorescence Blue Shift and Quantum Yield Enhancement,” J. Am. Chem. Soc. 136, 8269 (2014). DOI: 10.1021/ja5006866
Author affiliations: 1Texas A&M University, 2University of North Texas
Correspondence: * zhou@mail.chem.tamu.edu, ** omary@unt.edu
This work was supported as part of the Center for Gas Separations Relevant to Clean Energy Technologies, an Energy Frontier Research Center (EFRC) funded by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001015, and part of the Methane Opportunities for Vehicular Energy (MOVE) Program, an ARPA-E Project under Award Number DE-AR0000249. Z.W. and D.F. were supported by MOVE. Y.-P.C. and Z.-Y.G. were supported by EFRC. M.A.O. acknowledges support of his group’s contributions by the National Science Foundation (CHE-1413641; CHE-0911690; CMMI-0963509; CHE-0840518) and the Robert A. Welch Foundation (Grant B-1542). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.