Toxic vehicle emissions, such as carbon monoxide and unburned hydrocarbons, are chemically converted to benign compounds, like carbon dioxide (CO2) and water, by catalytic converters. Although catalytic converters are valuable technology, they are also expensive. To reduce costs, experiments are being done to lessen the amount of platinum (Pt) and substitute palladium (Pd) in converters. The U.S. Department of Energy Office of Science's Advanced Photon Source (APS) played a role in research that indicates a Pd/lanthanum (La)-alumina catalyst may result in a less-expensive catalytic converter with improved low-temperature carbon monoxide (CO) oxidation reactivity.
Catalytic converters, installed on vehicles with internal combustion and diesel engines, convert the toxic byproducts of combustion to less toxic compounds. In two-way (lean burn) converters, used primarily on diesel engines, CO and partially burned hydrocarbons are oxidized over a Pt or Pd catalyst to form CO2 and water. In addition, three-way (stoichiometric) converters, used on internal combustion engines, also reduce nitrogen oxides back to nitrogen. This is tremendously beneficial since nitrous oxide is 300 times more potent as a greenhouse gas than is CO2. Since diesel exhaust is cooler than gasoline exhaust, the two-way catalyst must perform at low temperatures and also survive severe exothermic reactions when the particulate filter in the catalyst regenerates.
The oxidizing and reducing catalysts in catalytic converters contain precious metals, especially the costly platinum group metals Pt and Pd; a Pt-Pd alloy is more effective than either metal alone. To keep costs down, manufacturers try to minimize the amount of catalyst required, or to use less expensive catalysts. The high cost of Pt has led to research to determine the hydrothermal stability of Pd, without the addition of Pt, particularly with La-alumina support.
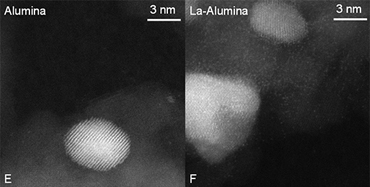
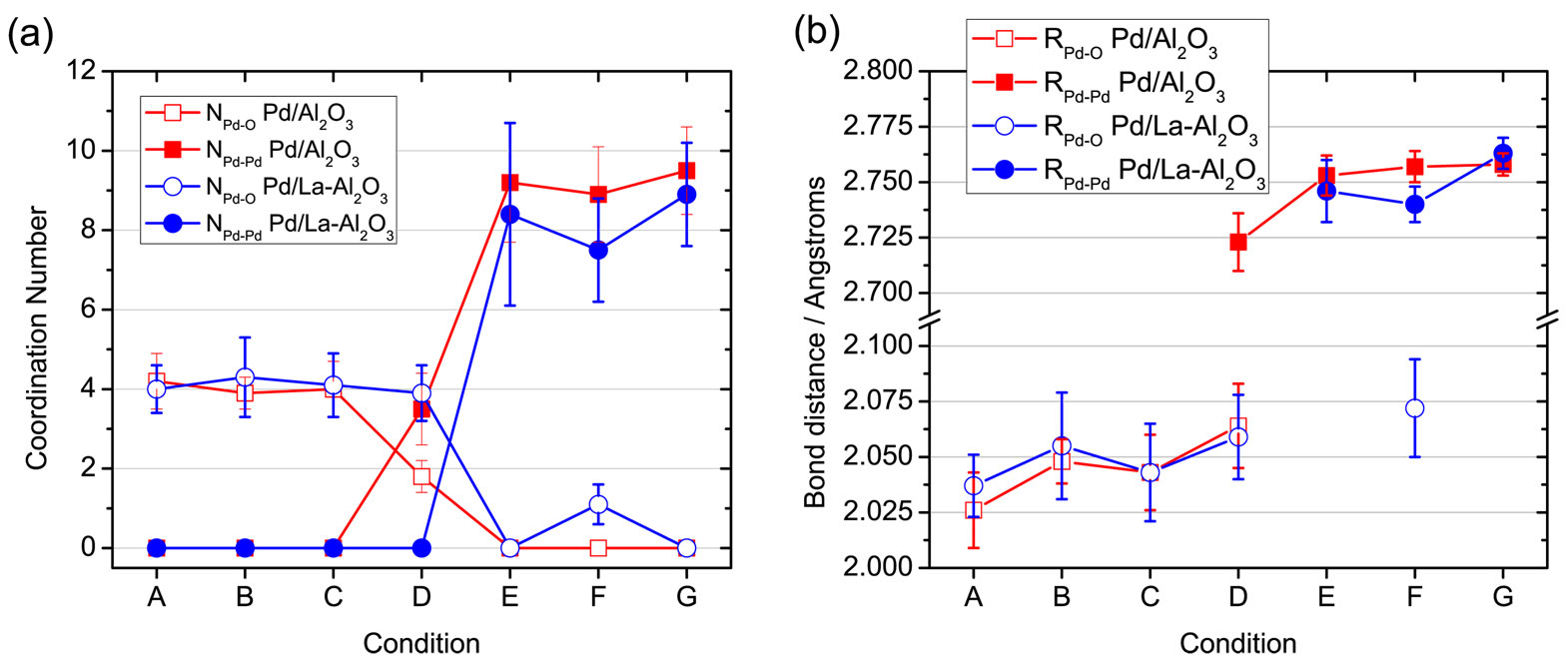
Researchers from the University of New Mexico and Argonne National Laboratory simulated diesel oxidation catalysts with 2.5% Pd and tested in two different preparations, one with alumina only and one with La-stabilized alumina. Each catalyst was roasted to 500° C to oxidize the Pd to PdO and then cooled to room temperature. When the temperature was raised to 140° C in the presence of CO, the PdO reduced to Pd metal. Results of the experiments were analyzed by x-ray absorption spectroscopy (XAS) and diffuse reflectance Fourier transform infrared spectroscopy (DRIFTS) at the X-ray Science Division 9-BM beamline at the Argonne National Laboratory Advanced Photon Source.
With the Pd-alumina catalyst, the absorption of CO was found to be irreversible because the catalyst had little reaction with oxygen. With the La-stabilized alumina, the Pd catalyst was more reactive toward oxygen and had higher CO conversion; CO oxidation began at a lower temperature, around 100° C. Pd became oxidized when the gas phase was switched from pure CO to the stoichiometric reaction mixture, and there was a reduction in the surface coverage of CO.The addition of La to the catalyst had two effects. First, Pd formed small particles and clusters, which increased Pd dispersion from 17% to 26%. The increase in dispersion is partially responsible for the improved CO conversion.
Second, the redox reaction was more likely at low temperature, so Pd was less susceptible to CO poisoning. The turnover frequency at 140° C improved 5-fold, due in part to the redox behavior of the Pd/La-alumina catalyst.
In catalytic converters, a Pt-Pd alloy is more stable, more reactive, and less susceptible to aging than Pt metal alone. Adding Pd in the alloy reduces the cost, hence the hydrothermal stability of Pd is important. The use of simultaneous XAS and DRIFTS in this research leads to a greater understanding of the effects of adding La to Pd-based catalysts in catalytic converters to improve the efficiency and decrease the costs of two-way catalytic converters used for diesel engines.
— Dana Desonie
See: Jason R. Gaudet1‡, Andrew de la Riva1, Eric J. Peterson1, Trudy Bolin2, and Abhaya K. Datye1*, "Improved Low-Temperature CO Oxidation Performance of Pd Supported on La-Stabilized Alumina," ACS Catal. 3, 846 (2013). DOI: 10.1021/cs400024u
Author affiliations: 1University of New Mexico, 2Argonne National Laboratory. ‡Present address: University of Michigan
Correspondence: *datye@unm.edu
This research was supported by the U.S. Department of Energy Office of Science Grant DE-FG02-05ER15712. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy Office of Science under contract no. DE-AC02-06CH11357. Electron microscopy was performed at EMSL, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy's Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.