Chemists who develop catalysts are always trying to improve catalytic efficiency or create novel reaction pathways, but they're doing so largely in the dark. The atomic-scale structure and chemical properties of catalysts remain surprisingly mysterious, despite the critical roles that catalysts play in a variety of industrial and environmental applications. Heterogeneous catalysts — which differ in phase from their substrates — are used, for example, to convert toxic nitric oxide from automotive emissions to less harmful gases.
Hoping to shed some light on how catalysts behave, and connect these behaviors to catalysts' activity and selectivity, researchers working at the U.S. Department of Energy Office of Science's Advanced Photon Source teased out structural and chemical information about a single layer of vanadium oxide, a catalyst, supported on the surface of a titanium oxide crystal. The data revealed that vanadium oxide undergoes a dramatic and reversible change in both chemical states and structure during a redox reaction cycle, providing unprecedented insight into how vanadium oxide catalyzes reactions. This research will make it possible for scientists to improve catalysts by strategically altering their structures.
Supported vanadium oxide is currently under investigation as a catalyst for the oxidative dehydration of ethanol, propane, and cyclohexane. For example, low-density arrangements of supported vanadium oxide have shown greater catalytic efficiency than supported vanadium oxide at higher densities or bulk vanadium oxide. In this study, researchers from Northwestern University, the Massachusetts Institute of Technology, and Argonne National Laboratory examined the oxidative dehydration of cyclohexane by monolayer vanadium oxide on a titanium oxide scaffold as a model system.
To make the sample, the researchers atomically engineered a layer of vanadium oxide on the surface of a single rutile (10 mm x 10 mm x 1 mm) titanium oxide crystal. The researchers created vanadium atomic density maps relative to the rutile substrate lattice using x-ray standing wave (XSW) in situ analysis at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) 5-ID-B,C,D beamline at the Argonne Advanced Photon Source.
The key to XSW is that it uses the x-rays generated by the APS beamline in two ways: single-crystal x-ray diffraction and x-ray fluorescence. The x-ray diffraction provides the basic structural outline, while the fluorescence distinguishes between vanadium and titanium.
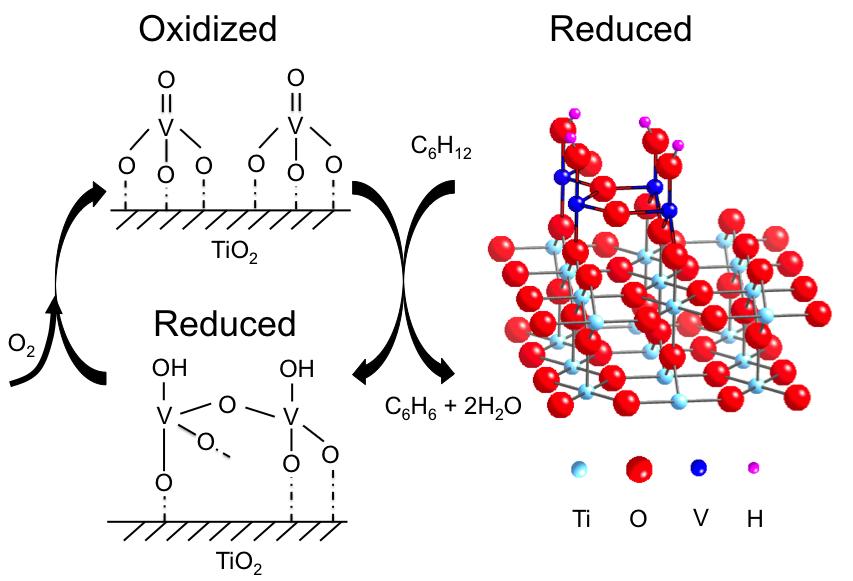
To run the in situ XSW experiments, the researchers placed the sample in the path of the x-ray beam inside a beryllium dome. The dome, which is "transparent" to x-rays, is used to create the environment for vanadium to catalyze chemical reactions, supplying the proper temperature, pressure, and reaction gases. To generate a three-dimensional image, the researchers rotated the crystal with a 5-circle diffractometer. They gathered structural data on the catalyst in three states: oxidized by oxygen, reduced by cyclohexane, and reoxidized by oxygen. This strategy can effectively track the behaviors of catalysts during a chemical reaction.
In addition to structural information, the researchers wanted to assess the chemical oxidation state of the catalyst under the same conditions as studied by XSW. For this, the researchers used a low-energy x-ray source provided by the Keck-II facility at Northwestern University.
X-ray photoelectron spectroscopy (XPS) detected the oxidation state of vanadium and any bound oxygen atoms under oxidized and reduced conditions. By combining the XPS and XSW data, the researchers developed an atomic-scale model for the reduced catalyst in a 4+ oxidation state (right in the figure). Its structure strongly resembles that of the titanium oxide support, which was expected, the researchers say, because titanium also has a 4+ oxidation state.
Things got more interesting when it came to the analysis of the oxidized state. This structure was uncorrelated to that of the underlying titanium oxide, indicating that the catalyst significantly changes upon oxidation. The disharmony between the oxidized vanadium oxide and titanium oxide support made it impossible for the researchers to develop a structural model for the oxidized state.
However, using the information from XPS data as well as from infrared spectroscopy, they figured out that oxidized vanadium is in a 5+ oxidation state, with three single bonds to oxygen and one double bond (figure, top left). The oxidized and reoxidized states looked the same, suggesting that the reaction is reversible. In future research, the researchers plan to nail down the oxidized structure by combining structural and oxidation state information.
— Erika Gebel Berg
See: Zhenxing Feng1,2, Junling Lu3, Hao Feng3, Peter C. Stair1, Jeffrey W. Elam3, and Michael J. Bedzyk1*, "Catalysts Transform While Molecules React: An Atomic-Scale View," J. Phys. Chem. Lett. 4, 285 (2013). DOI:10.1021/jz301859k
Author affiliations: 1Northwestern University, 2Massachusetts Institute of Technology, 3Argonne National Laboratory
Correspondence: *bedzyk@northwestern.edu
This work was supported by the Institute for Catalysis in Energy Processes (U.S. Department of Energy, Basic Energy Sciences, Chemical Sciences Grant DE-FG02-03ER15457). J.L. and J.W.E. were supported as part of the DOE, Office of Basic Energy Sciences, Chemical Sciences under the Hydrogen Fuel Initiative program. The DuPont-Northwestern-Dow Collaborative Access Team is supported by E.I. DuPont de Nemours & Co., The Dow Chemical Company and Northwestern University. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy Office of Science under Contract no. DE-AC02-06CH11357.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.