Innovative materials chemistries continue to drive advances in lithium-ion batteries-the state-of-the-art in rechargeable energy storage. While many different battery components contribute to their performance, the largest gains may be achieved through the development of new electrode materials that can power portable electronics longer and propel electronic vehicles farther. Electrodes based on so-called “conversion chemistry” have the potential to double energy storage capacities compared to electrodes in existing rechargeable batteries. This higher energy storage capacity is achieved because each metal atom can react with several electrons in conversion systems, compared to the single electron limit of conventional electrodes. Although these new materials initially deliver higher capacities, their capacities often diminish with repeated charging and discharging. Nevertheless, unusually good long-term performances, with consistently high capacities, have been found with mixed-anion systems such as oxyfluorides. Although they combine the favorable performance characteristics of simple oxides and fluorides, the electrochemical reactions giving rise to the improved performance of these more complex materials remain a mystery.
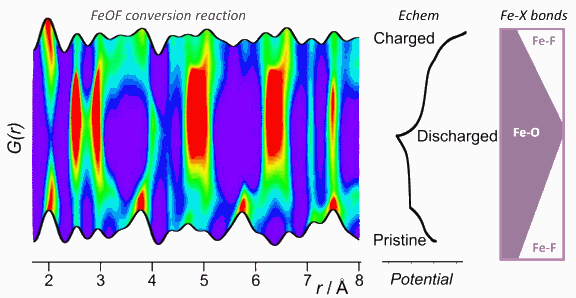
A team of scientists utilized the high-energy x-rays available at the U.S. Department of Energy Office of Science’s Advanced Photon Source (APS) at Argonne to investigate the fundamental basis for the performance advantage offered by mixed-anion iron oxyfluoride (FeOF) conversion electrodes, over electrodes made of simple oxide or fluoride phases. This research represents the first-of-a-kind application of operando pair distribution function (PDF) methods (that probe the structure of battery electrodes in situ while they are being cycled) to study electrochemical reactions in batteries. The PDF measurements provided exceptionally precise insight into the atom-atom bond distances, the proportion of each type of atom-atom bond, and how these evolve over dozens of points during the electrochemical reaction. This represents significant milestones in both understanding electrochemical reactions in battery electrodes and in the experimental tools available to investigate such reactions.
The scientists from Argonne National Laboratory, in collaboration with researchers at the University of Cambridge, Stony Brook University, Rutgers University, and CIC Energigune carried out their studies at X-ray Science Division (XSD) beamline 11-ID-B at the APS. A notable result was that the evolution of oxygen (O) and fluorine (F) species could be separated despite their nearly identical contributions to the x-ray measurements. These element-specific insights were recovered based on the distinct chemical bonding characteristics of these anions, with the bond lengths and coordination numbers within the electrodes being specified with high precision by the PDF data.
The PDF study (in combination with solid-state nuclear magnetic resonance spectroscopy) enabled the elucidation of a complex nanoscale transformation mechanism and provided new insights into the functionality of FeOF. Several critical insights emerged from the work that will impact the design of new battery materials:
• There is a preferential reaction of the fluoride component during both discharge and charge.
• The active electrode does not consist of the single-phase oxyfluoride of the pristine uncycled electrode, but rather contains multiple electrochemically active phases—an O-rich rock salt phase and a F-rich rutile phase (see the figure).
• Competition between electrochemical reactions involving the rock salt and rutile components in the active electrode and frustration in O/F ordering within these phases may favor the nanosized composite electrode structure that contributes to enhanced cyclability.
These results have important implications for producing other mixed-anion systems, suggesting a possible cost- and time-saving shortcut to achieving exemplary electrochemical performance. There may be no need to go to the trouble of preparing a single-phase oxyfluoride, because simply mixing an oxide with a fluoride could yield similar performance improvements, which is an entirely unexplored route for the development of new battery materials. This is particularly important in systems where oxyfluoride phases are not known (such as nickel).
The operando x-ray PDF studies were made possible by a newly developed electrochemical cell that is compatible with PDF measurements and a broad range of other x-ray scattering and spectroscopic methods. Argonne’s Multi-Purpose In-situ X-ray (AMPIX) electrochemical cell [1] provides reliable electrochemical cycling without compromising the x-ray measurement. The suitability of the AMPIX cell for a broad range of synchrotron-based x-ray scattering and spectroscopic measurements has been demonstrated with studies at eight APS beamlines to date. Compatible techniques include PDF analysis, high-resolution powder diffraction, small-angle scattering, and x-ray absorption spectroscopy. These techniques probe a broad range of electronic, structural, and morphological features relevant to battery materials.
The AMPIX cell enables experiments providing greater insight into the complex processes that occur in operating batteries by allowing the electrochemical reactions to be probed at fine reaction intervals with greater consistency (within the charge-discharge cycle and between different methodologies) with the potential for new time-dependent kinetic studies or studies of transient species. — Vic Comello
See: Kamila M. Wiaderek1, Olaf J. Borkiewicz1, Elizabeth Castillo-Martínez2,3, Rosa Robert2,4,‡, Nathalie Pereira5, Glenn G. Amatucci5, Clare P. Grey2,4, Peter J. Chupas1, and Karena W. Chapman1*, “Comprehensive Insights into the Structural and Chemical Changes in Mixed Anion FeOF Electrodes by Using Operando PDF and NMR Spectroscopy,” J. Am. Chem. Soc. 135(10), 4070 (March 13, 2013). DOI:10.1021/ja400229v
Author affiliations: 1Argonne National Laboratory, 2University of Cambridge, 3CIC Energigune, 4Stony Brook University, 5Rutgers University. ‡Present address: Paul Scherrer Institute
Correspondence: *chapmank@aps.anl.gov
This work was supported as part of NECCES, an Energy Frontier Research Center funded by the U.S. Department of Energy Office of Science (DOE-SC) under Award Number DE-SC0001294. Work done at Argonne and use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. DOE-SC by Argonne National Laboratory, were supported by the U.S. DOE-SC under Contract No. DE-AC02-06CH11357. The Generalitat de Catalunya is acknowledged for the postdoctoral research grant awarded to R. Robert (BP-DGR 2008).
Reference
[1] Olaf J. Borkiewicz, Badri Shyam, Kamila M. Wiaderek, Charles Kurtz, Peter J. Chupas, and Karena W. Chapman, “The AMPIX electrochemical cell: a versatile apparatus for in situ x-ray scattering and spectroscopic measurements,” J. Appl. Cryst. 45 (2012) 1261–1269. DOI:10.1107/S0021889812042720
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.