An unexpected magnetic behavior within Sr3CuIrO6, a transition-metal compound (TMC) that combines the transition metal copper with the transition metal iridium has been revealed by research at the U.S. Department of Energy Office of Science’s Advanced Photon Source (APS). These results indicate that mixing certain transition metal systems can yield TMCs with surprising physical properties unattainable with these systems alone, and may eventually lead to new materials for applications such as electronic memory devices and quantum computation.
The researchers in this study, published in Physical Review Letters, relied on theoretical calculations complimented by x-ray measurements performed at the X-ray Science Division 9-ID x-ray beamline at the Argonne National Laboratory APS to characterize the source of the unusual magnetism within Sr3CuIrO6.
Scientists are drawn to the unusual properties exhibited by TMCs (copper oxide, for instance, is a key ingredient in many high-temperature superconductors). These compounds typically contain oxygen and a transition metal. Transition metals reside in the middle portion of the Periodic Table of the Elements in groups 3 through 12. The interest in TMCs has largely focused on compounds containing transition metals with lower atomic numbers, specifically the elements scandium through zinc. These elements all lie in the third period of the table, and are collectively referred to as 3d transition metals (indicating their highest electron energy level).
Transition metals form the d-block within the periodic table, characterized by the unfilled electron d-sublevels of these elements. Iron, for instance, is a 3d transition metal. Since a full d-sublevel has 10 electrons, the 6 electrons in iron's d-sublevel means it is only partially filled. Elements in the d-block with higher atomic numbers (like iridium) possess additional electrons. For these elements the 3d-sublevel electrons stay much closer to the nucleus than those in the 5d-sublevel where the transition metal iridium is found.
This means the atom's 3d electrons experience a strong repulsive electrostatic interaction with the other 3d electrons, preventing them from hopping to neighboring atoms in TMCs, so the 3d electrons cannot freely move through the compounds and are said to be strongly correlated.
A primary process in TMCs featuring 3d transition metals like copper is the exchange of two electrons between two 3d-sublevel atoms, which does not change the number of 3d electrons on any 3d atom, avoiding the large penalty associated with the repulsive interaction. This exchange process, in which the physical quality that changes is not charge but electron spin or orbital orientation, explains the magnetic properties in 3d TMCs.
The highly-correlated electrons found in many compounds containing only 3d transition metals are absent in materials containing 4dand 5d transition metals. The spatially-extended orbitals of the 5d electrons weaken the repulsive electron-electron interaction and facilitate their hopping to neighboring atoms when incorporated into TMCs. This “Coulomb screening effect” prevents iridium and other 5d-based TMCs from employing exchange to achieve the type of strong electron correlation seen in 3d-based TMCs.
But recent research has shown that, in spite of Coulomb screening, a phenomenon called “spin-orbit coupling” (SOC) can impart significant electron correlation to 5d-based TMCs. For instance, spin-orbit coupling causes the 5d compound Sr2IrO4 to exhibit magnetic ordering similar to that of some 3d copper oxides. The strength of SOC is positively correlated with the size of the atom, so SOC is relatively negligible in 3d TMCs but is strong in noble metals and 5d TMCs. Its effects on magnetism in 5d TMCs are just beginning to be explored.
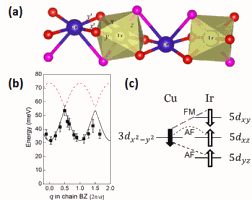
The key microscopic structure of the mixed 3d-5d Sr3CuIrO6 compound is depicted in (a) in the figure. This microscopic structure was probed using resonant inelastic x-ray scattering (RIXS), performed at beamline 9-ID. In the RIXS technique, highly-tuned x-rays are used to excite closely-bound electrons from the iridium cations onto a 5d-sublevel; when another 5d-sublevel electron transitions into the electron vacancy, a characteristic x-ray is emitted that provides information about the state of the material.
The x-ray data revealed excited magnetic states (magnons) within the Sr3CuIrO6 sample, characterized by a large spectral gap — (b) in the figure —which means that substantial energy is required to create a single magnon. This was unexpected since the material was modeled by a simple isotropic (uniform in all directions) Heisenberg model, implying a gapless magnon spectrum, so the observed large gap indicates the existence of strong anisotropy (direction-dependence). SOC is a natural way to account for the anisotropy because SOC breaks the rotational symmetry of the spins by coupling them to the d orbitals, which are directional. But this effect is usually small.
This unexpected magnetic anisotropy motivated the development of a new theory in which the antiferromagnetic and ferromagnetic exchanges mix, depending on which iridium-5d orbital is coupled with the copper-3d orbital. These different types of exchange interactions usually compete.
But amazingly, in the 3d-5d Sr3CuIrO6 compound they cooperate because the strong SOC on the iridium ion anti-parallelizes the orbital and spin angular momenta (c in the figure), leading to an effective ferromagnetic exchange between the copper's electronic spins and the iridium's total angular momentum, with the strong exchange anisotropy being induced by the antiferromagnetic exchange.
The promising results derived from this research are anticipated to open the way for the further development of mixed 3d-5dtransition metal systems.
“The direction of this exchange anisotropy depends on how the iridium and copper atoms are connected,” said Weiguo Yin of Brookhaven National Laboratory, lead author of the Physics Review Letters article. “By arranging the 3d and 5d atoms, we can develop advanced materials with specific spin orientations or spin frustration with the use of competing anisotropies.”
— Philip Koth
See: Wei-Guo Yin1*, X. Liu1,2, A. M. Tsvelik1, M. P. M. Dean1, M. H. Upton3, Jungho Kim3, D. Casa3, A. Said3, T. Gog3, T. F. Qi4, G. Cao4, and J. P. Hill1, “Ferromagnetic Exchange Anisotropy from Antiferromagnetic Superexchange in the Mixed 3d-5d Transition-Metal Compound Sr3CuIrO6”, Phys. Rev. Lett. 111, 057202 (2013). DOI:10.1103/PhysRevLett.111.057202
Author affiliations: 1Brookhaven National Laboratory, 2Chinese Academy of Sciences, 3Argonne National Laboratory, 4University of Kentucky
Correspondence: *wyin@bnl.gov
This work was supported by the U.S. Department of Energy (DOE), Division of Materials Science, under Contract No. DE-AC02-98CH10886. T.F.Q and G.C. were supported by the National Science Foundation through grant No. DMR-0856234. Use of the Advanced Photon Source is supported by the U.S. Department of Energy Office of Science under Contract No. DE-AC02-06CH11357.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.