Photosynthetic water oxidation is a fundamental process in the biosphere that results in the sunlight-driven formation of O2 from water. Biological photosynthesis encompasses a series of complicated processes involving several transition states and intermediates that scientists continue to investigate. Mimicking this reaction in a man-made device will allow for sunlight-to-chemical energy conversion, with water providing electrons and protons for the formation of oxygen and reduced chemicals, a process best suited for sustainable and clean generation of H2. The first synthetic catalyst designed to mimic the portion of biological photosynthesis involved in water oxidation, i.e., the catalyzed evolution of O2from H2O, was the ruthenium-based compound commonly referred to as “blue dimer” (BD). Although the water-oxidizing capabilities of blue dimer were first reported around three decades ago, several aspects of this catalytic process remained hidden. Recently, scientists utilizing a variety of spectroscopic techniques to probe the catalysis process, including x-ray absorption spectroscopy performed at X-ray Science Division beamline 20-BM at the U.S. Department of Energy Office of Science’s Advanced Photon Source at Argonne National Laboratory. Their results published in the Journal of the American Chemical Society report progress in revealing previously unknown mechanistic details about BD’s water oxidation reaction.
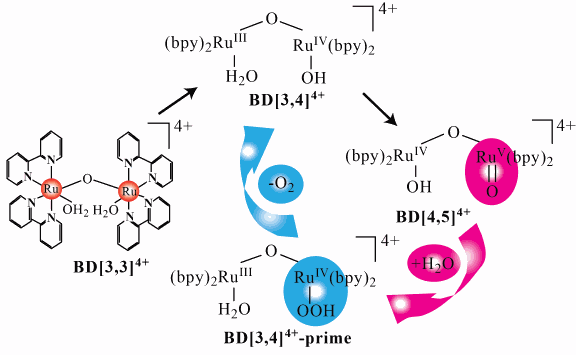
Blue dimer contains two ruthenium (Ru) atoms bound together by a single oxygen atom. Each Ru atom also forms bonds with a ligand. The ligands are composed of linked pyridine rings, structurally very similar to the simple benzene ring (C6H6). The two ruthenium atoms can exhibit different oxidation states. The chemical formula of blue dimer, cis,cis-[(bpy)2(H2O) RuIIIORuIII(OH2)(bpy)2]4+ (where “bpy” is 2,2-bipyridine), indicates that the two ruthenium atoms are in the [3,3] oxidation state. A water molecule is attached to each Ru atom in the [3,3] state.
The researchers from Purdue University, the University of North Carolina at Chapel Hill, and Southern Federal University (Russia) monitored the catalytic cycle of BD via stopped-flow ultraviolet-visible spectroscopy with millisecond precision, electron paramagnetic resonance (EPR), resonance Raman spectroscopy, and x-ray absorption spectroscopy (XAS). Use of techniques with high sensitivity to the electronic states of molecules, namely EPR and XAS, was crucial in determining the electronic requirements of the water oxidation process. Extended x-ray absorption fine structure (EXAFS) measurements allowed determination of bond distances. This research identified for the first time structural details of two short-lived intermediates involved in the BD catalytic cycle, one reactive towards the formation of the O-O bond and other, the product of this reaction (peroxo-intermediate).
The experimental results revealed new aspects about the intermediates and time frames utilized by the BD catalyst to oxidize water. Research shows that under studied conditions, and in spite of fast reaction with water, evolution of oxygen lagged behind. This was attributed to stabilization of the peroxo species. This new information should help the development of the next generation of synthetic catalysts from the water oxidation reaction. These should form a highly oxidized state analogous to the RuV = O species with good reactivity toward water, and aid in the conversion of peroxo species resulting from this reaction. The researchers hope that their findings will ultimately lead to practical solutions in the quest to find a cost effective, practical, and sustainable alternative energy sources. — Philip Koth
See: Dooshaye Moonshiram1, Jonah W. Jurss2, Javier J. Concepcion2, Taisiya Zakharova1, Igor Alperovich2,3, Thomas J. Meyer2, and Yulia Pushkar1*, “Structure and Electronic Configurations of the Intermediates of Water Oxidation in Blue Ruthenium Dimer Catalysis,” J. Am. Chem. Soc. 134, 4625 (2012). DOI:10.1021/ja208636f
Author affiliations: 1Purdue University, 2University of North Carolina at Chapel Hill, 3Southern Federal University
Correspondence: *ypushkar@purdue.edu
D.M. was partially supported by a Purdue Research Foundation grant. We thank the U.S. Department of Energy (DOE) Office of Science Basic Energy Sciences Program for financial support of this work under grants DE-FG02-10ER16184 (Y.P.) and DE-FG02-06ER15788 (T.M.). Synthesis and characterization (J.J.C.) were supported by the UNC EFRC: Solar Fuels and Next Generation Photovoltaics, an Energy Frontier Research Center funded by the U.S. DOE Office of Science, Basic Energy Sciences, under Award Number DE-SC0001011. Utilization of 20-BM-B supported by DOE Basic Energy Sciences, a Major Resources Support grant from the Natural Sciences and Engineering Research Council of Canada, the University of Washington, the Canadian Light Source, and the APS. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the DOE Office of Science under Contract No. DE-AC02-06CH11357.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.