Observing the evolution of a particular type of antibody in a patient infected with HIV-1, the most common and pathogenic strain of the human immunodeficiency virus (HIV), has provided insights that will enable vaccination strategies that mimic the actual antibody development within the body. Spearheaded by Duke University, the multi-institution study included analysis from the U.S. Department of Energy (DOE) National Nuclear Security Administration’s Los Alamos National Laboratory and used high-energy x-rays from the DOE Office of Science’s Advanced Photon Source (APS) at Argonne National Laboratory.
The kind of antibody studied is called a broadly cross-reactive neutralizing antibody, and details of its generation could provide a blueprint for effective vaccination, according to the study’s authors. In a paper published in Nature, the team reported on the isolation, evolution, and structure of a broadly neutralizing antibody from an African donor followed from the time of infection.
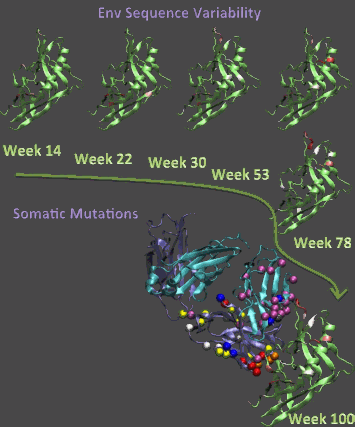
The observations trace the co-evolution of the virus and antibodies, ultimately leading to the development of a strain of the potent antibodies in this subject, and they could provide insights into strategies to elicit similar antibodies by vaccination.
Patients early in HIV-1 infection have primarily a single “founder” form of the virus that has been strong enough to infect the patient, even though the population in the originating patient is usually far more diverse and contains a wide variety of HIV mutations. Once the founder virus is involved in the new patient’s system, the surrounding environment stimulates the HIV to mutate and form a unique, tailored population of virus that is specific to the individual.
The research team, including Bette Korber, Peter Hraber, and S. Gnanakaran of Los Alamos National Laboratory, and led by Barton Haynes of Duke University School of Medicine, with colleagues at Boston University, the National Institutes of Health (NIH), Stanford University, Columbia University, Kamuzu Central Hospital, and the University of Pennsylvania showed that broadly neutralizing antibodies developed only after the population of viruses in the individual had matured and become more diverse.
“Our hope is that a vaccine based on the series of HIV variants that evolved within this subject, that were together capable of stimulating this potent broad antibody response in his natural infection, may enable triggering similar protective antibody responses in vaccines,” said Bette Korber, leader of the Los Alamos team.
Article co-authors Peter Kwong and Tongqing Zhou from the Vaccine Research Center, NIH, lead the x-ray crystallographic studies of the antibodies and virus with the goal of creating an atom-by-atom picture of the co-evolution of the antibody CH103 and the HIV-1 virus. An atomic-level picture of what induces the body to create antibodies that can neutralize more than one strain of the virus is crucial to creating a vaccine that can stay ahead of the virus as it mutates in the body. The intensely focused x-rays of the APS, utilized at the Southeast Regional Collaborative Access Team (SER-CAT) 22-ID x-ray facility, allowed the researchers to hone in on a 50-µm-sized crystal containing the epitope of the HIV virus that is recognized by the body¹s immune system and the broadly neutralizing antibody it develops. This interaction could only be seen using brilliant x-rays such as those at the APS because the scientists could not grow larger crystals of the antibody-virus complex.
See: Hua-Xin Liao1,2*, Rebecca Lynch3, Tongqing Zhou3, Feng Gao1,2, S. Munir Alam1,2, Scott D. Boyd4, Andrew Z. Fire4, Krishna M. Roskin4, Chaim A. Schramm5, Zhenhai Zhang5, Jiang Zhu3, Lawrence Shapiro3,5, James C. Mullikin3, S. Gnanakaran6, Peter Hraber6, Kevin Wiehe1,2, Garnett Kelsoe1,2, Guang Yang1,2, Shi-MaoXia1,2, David C. Montefiori1,2, Robert Parks1,2, Krissey E. Lloyd1,2, Richard M.Scearce1,2, Kelly A. Soderberg1,2, Myron Cohen7, Gift Kamanga8, Mark K. Louder3, Lillian M. Tran3, Yue Chen1,2, Fangping Cai1,2, Sheri Chen1,2, Stephanie Moquin3, Xiulian Du3, M. Gordon Joyce3, Sanjay Srivatsan3, Baoshan Zhang3, Anqi Zheng3, George M. Shaw9, Beatrice H. Hahn9, Thomas B. Kepler10, Bette T.M. Korber6, Peter D. Kwong3, John R. Mascola3, and Barton F. Haynes1,2**, “Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus,” Nature, published online 03 April 2013.
Author affiliations: 1Duke University School of Medicine, 2Duke Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery, 3National Institutes of Health, 4Stanford University, 5Columbia University, 6Los Alamos National Laboratory, 7University of North Carolina, 8Kamuzu Central Hospital, 9University of Pennsylvania, 10Boston University
Correspondence:* hliao@duke.edu, **barton.haynes@duke.edu
This study was supported by the National Institutes of Allergy and Infectious Diseases (NIAID) and by intramural National Institutes of Health (NIH) support for the NIAID Vaccine Research Center, by grants from the NIH, NIAID, AI067854 (the Center for HIV/AIDS Vaccine Immunology) and AI100645 (the Center for Vaccine Immunology-Immunogen Discovery). SER-CAT is an organization consisting of 22 member institutions, formed in 1997 to provide third-generation x-ray capabilities to macromolecular crystallographers and structural biologists in the southeastern region of the U.S. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the DOE Office of Science under Contract No. DE-AC02-06CH11357.
The original Los Alamos National Laboratory news release by Nancy Ambrosiano can be read here.
The Argonne news story by Nancy Ambrosiano and Tona Kunz (Argonne) can be read here.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science X-ray user facilities, visit the user facilities directory.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
Los Alamos National Laboratory, a multidisciplinary research institution engaged in strategic science on behalf of national security, is operated by Los Alamos National Security, LLC, a team composed of Bechtel National, the University of California, The Babcock & Wilcox Company, and URS for the Department of Energy’s National Nuclear Security Administration. Los Alamos enhances national security by ensuring the safety and reliability of the U.S. nuclear stockpile, developing technologies to reduce threats from weapons of mass destruction, and solving problems related to energy, environment, infrastructure, health, and global security concerns.