A colloidal suspension is a mixture in which microscopic particles of one substance are dispersed in another and because of its properties does not “settle” in the way that one might expect a stirred mixture of sand in water to do; nor do the particles dissolve in the fluid as one might expect grains of salt to dissolve in water. Colloidal suspensions and the related gels (solid colloidal systems) are of interest because many of them have fundamentally useful properties. Natural systems such as milk, the interior of cells, even atmospheric fog are colloidal systems. Synthetic colloids exist in coatings, cosmetics, and elsewhere.
Understanding colloids could lead to a deeper understanding of complex soft matter, with implications for new or improved materials and polymer science, but we have only an incomplete picture of the structure and dynamics of colloidal suspensions.
The researchers in this study, at the National Institute of Standards and Technology, Northern Illinois University, and Argonne National Laboratory, suggest that concentrated colloidal suspensions of microspheres in a liquid are, apparently, not simple systems.
Colloidal suspensions never “settle” because the unceasing collisions among the molecules — Brownian motion — that form the suspending fluid and the colloidal particles counteract the effect of gravity on their greater density; they never sink.
If one swirls grains of sand in a beaker of water, the thermal motions of the water molecules will not be sufficient to prevent gravity from causing the sand to eventually form sediment at the bottom of the beaker once it is left to stand for long enough. However, microscopic particles of sand in the mix might remain suspended indefinitely.
Working with high-brightness x-rays from the U.S. Department of Energy Office of Science’s Advanced Photon Source (APS) at Argonne, the research team used the new synchrotron x-ray technique called ultra-small-angle x-ray scattering (USAXS)–x-ray photon correlation spectroscopy (XPCS) to help them see through colloidal systems in order to reveal inner secrets. This new technique, implemented on the dedicated USAXS beamline (initially the X-ray Science Division 32-ID beamline, and later the ChemMatCARS 15-ID beamline) at the APS, overcomes the problem of attempting to use light to study such opaque systems because the wavelengths of x-rays are so much shorter than that of visible light and so can resolve details of the particles involved and their behavior on concomitantly shorter length scales.
The team points out that this new approach also overcomes several of the technical limitations of conventional XPCS, allowing larger distances to be observed and so provides data on rapidly moving microspheres. Fundamentally, they can observe particles tenths of a micrometer to several micrometers in diameter.
The team worked with microscopic spheres of the common polymer, polystyrene, and suspended these particles in the colorless and viscous fluid glycerol, which is technically a sugar alcohol compound and is used widely in cosmetics and pharmaceutical formulations. They suspended a range of volumes of polystyrene spheres — from 10% to 20% — in the fluid and used USAXS−XPCS to monitor the motions of the spheres in the fluid.
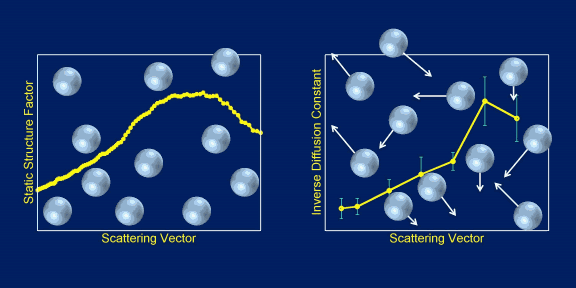
Their key finding is that the microscopic polystyrene spheres move in a way rather different from what had been suspected, based on the simple notion of the glycerol molecule buffeting them and preventing them from settling (see the figure).
Rather than the apparently simple Brownian motion taking place in this colloidal suspension, it seems that the microspheres move collectively, whereby buffeted particles pull along their neighbors. Such behavior implies that, compared with expectations, the suspended particles spend much longer times in close proximity without touching. Potentially, this behavior could be exploited in cases where suspended particles serve as centers for chemical reactions.
Future experimental work is aimed at improving the time resolution of the measurement so that the more industrially and environmentally important aqueous systems can be studied. — David Bradley
See: Fan Zhang1,3*, Andrew J. Allen1, Lyle E. Levine1, Jan Ilavsky2, and Gabrielle G. Long1,2, “Structure and Dynamics Studies of Concentrated Micrometer-Sized Colloidal Suspensions,” Langmuir 29, 1379 (2013). DOI: 10.1021/la3044768
Author affiliations: 1National Institute of Standards and Technology, 2Argonne National Laboratory, 3Northern Illinois University
Correspondence: *fan.zhang@nist.gov
ChemMatCARS Sector 15 is principally supported by the National Science Foundation/Department of Energy under grant number NSF/CHE-0822838. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy Office of Science under contract no. DE-AC02-06CH11357.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.