The nucleic acid RNA is an essential part of the critical process by which the cells in our bodies manufacture proteins. But noncoding RNAs also exist whose sequences, while not converted into proteins, play important roles in many biological processes.
RNA molecules aggregate into complex three-dimensional (3-D) or “tertiary” structures, producing globular forms stabilized by various interactions. Proteins, ligands, and other RNA molecules recognize these folded RNAs and result in the biochemical pathways that affect all aspects of cellular metabolism.
Utilizing synchrotron x-ray scattering at the Biophysics Collaborative Access Team (Bio-CAT) beamline 18-ID at the Argonne Advanced Photon Source (APS), researchers investigated the unique folding behavior of ribozyme, which is an RNA that acts as a catalyst. Their work provides a path for predicting the structures of newly discovered noncoding RNAs, and will ultimately enhance understanding of how noncoding RNAs take on important biological functions.
RNA is one of two types of nucleic acids found in all cells. Its main role is to carry instructions for protein synthesis from DNA, the second type of nucleic acid, which stores the genetic information in cells. While messenger RNA represents the RNA that codes for proteins, noncoding RNAs also exist that are not translated into proteins. Noncoding RNAs are found widely in biology, having roles in the process of protein translation or gene regulation, for example.
It is now thought that noncoding RNAs play a role in even more biochemical functions than originally suspected. To do this, noncoding RNAs must adopt unique, complex 3-D structures that are critical to their function - creating sites that allow for chemical reactions or control gene expression.
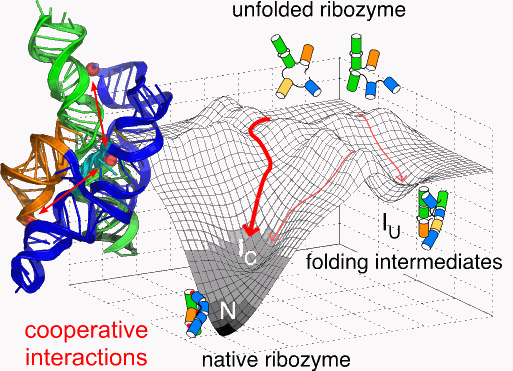
The tertiary structure of RNA refers to the 3-D arrangement of RNA building blocks that are held together via connections known as tertiary interactions. Although studies of noncoding RNAs have revealed the existence of structural themes known as tertiary motifs, the exact mechanism by which these encode the self-assembly of unique 3-D RNA structures remains poorly understood. This study aimed to examine why RNAs fold so specifically in spite of the relatively small number of tertiary motifs.
Small-angle x-ray scattering (as well as other techniques) at Bio-CAT beamline 18-ID at the U.S. Department of Energy’s APS allowed the researchers, from Johns Hopkins University, the University of Maryland, and the National Institute of Standards and Technology to measure changes in the folding energy landscape, showing that these tertiary interactions are highly related to the folding intermediates of the ribozyme.
A key finding was that the formation of structural motifs is cooperatively linked in near-native folding intermediates, and this cooperativity depends on the native helix orientation. The research team demonstrated how this cooperativity occurs early in the RNA folding process.
Coupling between tertiary structures in different areas of the RNA inhibits nonnative structures, while favoring the active RNA structure by increasing the free energy gap between the native state and the next most stable structure, thus simplifying the search for the native fold.
The researchers determined how stabilizing tertiary interactions cooperate at an intermediate stage of folding, much earlier in the folding process than previously suspected. The native structure of ribozyme was found to be important for this cooperation, with small alterations in the ribozyme architecture determining the entire folding pattern.
The study shows that tertiary interactions are very important at early stages of folding, but they have surprisingly little effect on the further stability of the native state of the ribozyme. While cooperation between tertiary interactions at different places in the RNA is important for getting from the unfolded to the intermediate state, this is less important for getting from the intermediate state to the native state.
This research has provided crucial insights about the importance of these early interactions in the RNA folding process, and indicates that cooperativity in noncoding RNAs may have arisen as an evolutionary process due to natural selection of structures that favor formation of unique folds.
The results increase our understanding of tertiary interactions in RNA and how they promote cooperative self-assembly, and will guide further research into the components of tertiary RNA structure, helping to predict the structures of newly discovered noncoding RNAs, and ultimately enhancing our understanding of these important biological functions. — Nicola Parry
See: Reza Behrouzi1, Joon Ho Roh2,3, Duncan Kilburn1, R.M. Briber2, and Sarah A. Woodson1*, “Cooperative Tertiary Interaction Network Guides RNA Folding,” Cell 149, 348 (April 13, 2012). DOI:10.1016/j.cell.2012.01.057
Author affiliations: 1Johns Hopkins University, 2University of Maryland, 3National Institute of Standards and Technology
Correspondence: *swoodson@jhu.edu
This work was supported by grants from the National Institutes of Health (NIH) (GM60819) and National Institute of Standards and Technology. Bio-CAT is a member of Illinois Institute of Technology's Center for Synchrotron Radiation Research and Instrumentation and is funded by the NIH.
Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy’s Office of Science under Contract No. DE-AC02-06CH11357.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.