Nature makes science fun by never failing to surprise.
Just when scientists think they have a thing figured out, nature sends them scurrying to the whiteboard because of some unexpected result.
The latest involves the so-called “bond-valence model,” which for years has been used to relate the number of nearest neighbors of the central atom of molecule or crystal (coordination number) and the interatomic distances between the atoms to the valence state.
“Valence” represents the number of bonds that an atom can form with one or more other atoms. The bond-valence sum rules provided an easy way to calculate valence from the coordination number and interatomic distances.
Research at the U.S. Department of Energy Office of Science’s Advanced Photon Source (APS) by researchers from Argonne National Laboratory, the Brazilian Synchrotron Light Laboratory, the Carnegie Institution of Washington, and Commissariat à l'énergie atomique-CEA (France) has shown, however, that gaining valence information requires a lot more work when it comes to mixed-valence systems. Their results have been published in the journal Physical Review Letters.
The culprit that caused all this trouble was Europium monoxide (EuO), which is known to be a bit unruly even under ideal circumstances. Under ambient conditions, EuO has the same crystal structure as common table salt, sodium chloride (NaCl), and, as with salt, the two atoms inside each molecule exist as positively and negatively charged ions.
The Eu ion usually has a +2 valence (Eu+2) under ambient conditions, but the Eu+3 valence state is energetically close by, meaning that it wouldn’t take much to induce a shift to the Eu+3 valence state. That’s because Europium’s valence state is not primarily due to the electrons occupying a single orbital level inside the atom, as is true of less obstreperous materials, but to the combined effect of electrons from different orbits. This is what makes EuO a mixed-valence system.
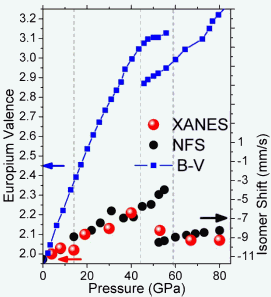
The scientists in this study loaded polycrystalline samples of EuO into a diamond anvil cell and performed x-ray diffraction (XRD) on APS x-ray beamline 16-BM-D (operated by the High Pressure Collaborative Access Team), and x-ray absorption near-edge spectroscopy (XANES) and nuclear forward scattering (NFS) experiments under high pressure on Argonne X-ray Science Division beamlines 4-ID-D and 3-ID-D, respectively. The XRD experiments allowed the scientists to determine how EuO volume changed upon being squeezed by pressures as high as 92 gigapascals (GPa), which is more than 13 million pounds per square inch. The XANES measurements directly probed the electronic structure of EuO as a function of lattice contraction, thus providing valence information. The pressure dependence of Eu valence was verified using the NFS technique.
The XRD data indicated that the EuO volume decreased steadily with increasing pressure until a pressure of about 45 GPa was reached, where a sudden 7% volume collapse was seen. From there on, the volume decreased continuously once again.
“Pressurizing a material usually causes a gradual decrease in volume, which tails off at high pressures because core electrons in atoms repel each other,” said lead author Narcizo Souza-Neto, a former postdoctoral researcher at the APS who is currently a scientist at the LNLS. “Sudden volume changes, however, are rare and require a different explanation.”
The volume collapse was found to be due to a shift in crystal structure from the NaCl structure to the cesium chloride (CsCl) structure, which has a higher coordination number and a longer bond length. This much was well known, leading people to speculate that the Eu2+ ions changed into Eu3+ ions at the same time.
“The ionic size of Eu3+ is about 20% smaller than Eu2+,” Souza-Neto said. “Since the Eu3+ state is close to the Eu2+state in energy, people have assumed that the volume collapse is accompanied, and possibly driven, by a valence transition to the Eu3+ state. We found that this is not the case.”
The researchers found that Europium ions remained in the Eu2+ state until a pressure of 14 GPa was reached. Between 14 and 44 GPa, the Europium ions displayed a mixed-valence state composed of discrete and fluctuating Eu2+ and Eu3+ ions that were distributed homogeneously throughout. NaCl and CsCl structures coexisted from 44 GPa to 59 GPa, with the Europium ions in the CsCl structures displaying a nearly Eu2+ state. Above 59 GPa, the CsCl structure was seen throughout, with the Europium ions being in the Eu2+ state.
“The Eu ions do initially move toward a 3+ state as the pressure goes up, but the volume collapse is accompanied by a decrease in valence and an increase in ion size, which the CsCl structure allows, since it gives the Eu ions a bit more room to expand, permitting them to revert to the 2+ valence,” said Argonne physicist Daniel Haskel, a collaborator on the experiment.
“The numerous superb facilities for high-pressure research at the APS allowed us to get a full picture of this electronic transition. As it turns out, the bond-valence model cannot deal with the fluctuating valence state observed up to 45 GPa since the dynamics of this fluctuation are too fast for the lattice to respond, causing the relationship between valence and bond distance to break down,” Haskel said.
Europium monoxide may not be found in the typical household today, but that may soon change. Because of its properties as a spin-polarized ferromagnetic semiconductor, much research is being directed toward manufacturing EuO spintronic devices. Spintronics is the emerging technological field that has already given us high-capacity computer hard drives based on the giant magnetoresistance effect.
— Vic Comello (VComello@anl.gov)
See: Narcizo M. Souza-Neto1,2,*, Jiyong Zhao1, Ercan E. Alp1, Guoyin Shen3, Stas V. Sinogeikin3, G. Lapertot4, and Daniel Haskel1,**, “Reentrant valence transition in EuO at high pressures: beyond the bond-valence model,” Phys. Rev. Lett. 109, 026403 (2012). DOI:10.1103/PhysRevLett.109.026403
Author affiliations: 1Argonne National Laboratory, 2 Brazilian Synchrotron Light Laboratory, 3Carnegie Institution of Washington, 4CEA-France
Correspondence: *narcizo.souza@lnls.br, **haskel@aps.anl.gov
See also: Science magazine Editors’ Choice, Physics, “Bond Valence Under Pressure,” by Jelena Stajic
Work at Argonne is supported by the U.S. Department of Energy (DOE) Office of Science under Contract No. DE-AC-02- 06CH11357. The High Pressure Collaborative Access Team is supported by the Carnegie Institution of Washington, the Carnegie DOE Alliance Center, the University of Nevada, Las Vegas, and Lawrence Livermore National Laboratory through funding from DOE- National Nuclear Security Administration, DOE- Basic Energy Sciences and the National Science Foundation.
Use of the Advanced Photon Source at Argonne National Laboratory was supported by the DOE’s Office of Science under Contract No. DE-AC02-06CH11357.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.