New information that could help in the fight against asthma has been obtained by an international collaboration of scientists utilizing the U.S. Department of Energy’s Advanced Photon Source at Argonne National Laboratory. Their results, which were recently published in the journal Nature, show how an important human transmembrane protein functions at a molecular level. The findings are significant in that the particular human transmembrane protein known as β2-adrenergic receptor, a G protein-coupled receptor (GPCR), is the focus of a series of drugs for the treatment of asthma. This new research on its structure and function has the potential of leading to the development of improved drug therapies.
There are over 750 human GPCRs distributed throughout the body with representatives in almost every cell type. They function in myriad ways enabling us to interact with our environment, and with one another through the sense of sight and smell. They also play key roles in heart and lung function, in how we respond to hormones and neurotransmitters and by extension how they influence mood and behavior, and are involved in immunity and inflammation. It is apparent, therefore, why a full suite of properly functioning GPCRs is integral to human health and wellbeing. About a third of drugs on the market today target GPCRs.
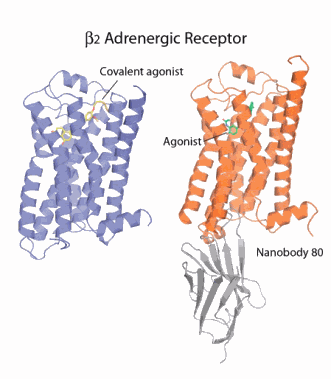
The research, by investigators from the Stanford University School of Medicine, Trinity College Dublin, the University of Limerick, Friedrich Alexander University, D. E. Shaw Research, the University of Michigan Medical School, and the University of Wisconsin-Madison set out to understand how one of these GPCRs, the β2-adrenergic receptor, works at a molecular level. The receptor is long enough to comfortably span the 5-nanometer width of the cell’s outer protective membrane. In this way, one end of the receptor can sense what is happening outside the cell and transmit the information it collects there to the cell’s interior for appropriate action. The fight-or-flight hormone adrenaline mediates its activity by way of the β2-adrenergic receptor. When the receptor binds adrenaline shot into the blood by the adrenal gland it snaps into action by binding with its cognate G-protein located inside the cell. This interaction triggers a series of reactions within and between cells that are part of the body’s response to adrenaline. These include vasodilation and constriction, smooth muscle relaxation, hearth muscle contraction, and mobilization of energy reserves in the liver and muscle. The β2-adrenergic receptor is an important pharmaceutical paradigm for the larger family of GPCRs, some of which are targeted by beta blockers.
Martin Caffrey, Trinity College Professor of Membrane Structural and Functional Biology in the Schools of Medicine and Biochemistry & Immunology explained: “New and improved drugs are always in demand. But to design them in a rational way we need to know how the receptor, in this case the β2-adrenergic receptor, is put together in a structural sense and how this structure enables it to function as a receptor and a communicator of information.
“By structure we refer to the arrangement in three-dimensions of the receptor’s constituent atoms, amino acids, and the ligands it binds. We would also like to know how this structure changes when adrenaline nudges the receptor and how this facilitates downstream signaling.
“The only way to get such detailed information for a complicated membrane protein like the β2-adrenergic receptor is to use macromolecular crystallography, which requires a well-ordered crystal of the receptor. The crystals must then be irradiated with x-ray photons in a way that can be used to decipher the receptor’s structure.
“One of the big challenges in this protracted and involved process is to coax the protein into the regular and ordered lattice of a crystal,” said Caffrey. “This is particularly difficult in the case of GPCRs because they continually flit about structurally in the plane of the membrane and, at any one moment, can be seen to exist in a number of different conformations or shapes. The particular conformation assumed, in turn, dictates the receptor’s biological activity. To get a collection of receptor molecules to crystallize, ideally they should all assume the one stance or conformation.”
Brian Kobilka, Professor of Molecular and Cellular Physiology at Stanford University, who led this research project, devised a strategy for locking the receptors into what amounts to a single conformation by covalently or irreversibly splicing an adrenaline look-alike molecule into the binding site of the receptor. So stabilized and rendered uniform, the receptor was successfully crystallized and its structure solved.
The study featured the use of a novel, high-throughput method and instrumentation, developed by the Trinity team members that crystallized the stabilized β2-adrenergic receptor. Custom-designed robots dispensed nanoliter volumes of a highly viscous, protein-laden lipidic liquid crystal or mesophase into home-built, multi-well glass sandwich plates for crystallization screening. The mesophase mimics the lipid bilayer membrane in which the receptor resides in the cell. When treated appropriately it undergoes a transition to a second mesophase in which receptor molecules preferentially cluster, arrange themselves regularly in two- and then three-dimensional arrays, and eventually form crystals. The crystals typically are very small, just a tenth to a hundredth of a millimeter in size, and are extremely fragile. They were harvested carefully from the toothpaste-textured mesophase, cryo-cooled in liquid nitrogen, and then shipped in special dewars from the laboratory at Trinity to the General Medicine and Cancer Institutes Collaborative Access Team facility on x-ray beamline 23-ID at the Advanced Photon Source. There, the team used state-of-the-art technologies to center the crystal in the x-ray beam and collect diffraction data, which, upon processing and modeling by researchers at Stanford, generated the structure reported in Nature.
See: Daniel M. Rosenbaum1‡, Cheng Zhang1, Joseph A. Lyons2,3, Ralph Holl4, David Aragao3, Daniel H. Arlow5, Søren G. F. Rasmussen1, Hee-Jung Choi1,6, Brian T. DeVree7, Roger K. Sunahara7, Pil Seok Chae8, Samuel H. Gellman8, Ron O. Dror5, David E. Shaw5, William I. Weis1,6, Martin Caffrey3**, Peter Gmeiner4***, and Brian K. Kobilka1*, “Structure and function of an irreversible agonist-β2 adrenoceptor complex,” Nature 469, 236 (13 January 2011). DOI:10.1038/nature09665
Author affiliations: 1Stanford University School of Medicine, 2University of Limerick, 3Trinity College, 4Friedrich Alexander University, 5D. E. Shaw Research, 6Stanford University School of Medicine, 7University of Michigan Medical School, 8University of Wisconsin-Madison. ‡Present address: University of Texas Southwestern
Correspondence: * kobilka@stanford.edu, ** martin.caffrey@tcd.ie, or
** peter.gmeiner@medchem.uni-erlangen.de
The research was supported by National Institutes of Health Grants NS028471 andGM083118 (B.K.K.),GM56169 (W.I.W.), P01GM75913 (S.H.G), GM75915 and P50GM073210 (M.C), and P60DK-20572 (R.K.S.); the Mathers Foundation (B.K.K and W.I.W); the Lundbeck Foundation (Junior Group Leader Fellowship, S.G.F.R.); Science Foundation Ireland (07/IN.1/B1836) and FP7 COST Action CM0902 (M.C.); the Bavaria California Technology Center (P.G.); and the
University of Michigan Biomedical Sciences Scholars Program (R.K.S).
Use of the Advanced Photon Source was supported by the U. S. Department of Energy Office of Science, under Contract No. DE-AC02-06CH11357.
See also: Søren G. F. Rasmussen et al., “Structure of a nanobody-stabilized active state of the β2 adrenoceptor,” Nature 469, 175 (13 January 2011). DOI:10.1038/nature09648
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science. The APS is the source of the Western Hemisphere’s brightest high-energy x-ray beams for research in virtually every scientific discipline. More than 3,500 scientists representing universities, industry, and academic institutions from every U.S. state and several foreign nations visit the APS each year to carry out applied and basic research in support of the BES mission to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels in order to provide the foundations for new energy technologies and to support DOE missions in energy, environment, and national security.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.