Sometimes size does matter, such as when certain nanomaterials exhibit different behaviors under varying extreme conditions. An example is yttrium oxide (Y2O3), a compound employed in industrial coatings and many other applications, including nanotechnology. But as with many similar materials, the qualities and stabilities of yttrium oxide on the nanoscale can vary greatly from those of the bulk material, so a thorough understanding of those differences and how they work is crucial. An experimental team working at the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne National Laboratory has discovered that Y2O3 in nanometer-sized particles undergoes a definite phase transition under pressure that results in characteristics quite different from bulk Y2O3, a finding with important implications for the use of yttrium oxide as a nanomaterial. Similar transitions have also been observed in silicon dioxide and other bulk materials, but the exact mechanism of the amorphization process has remained elusive.
Led by the HPSynC (the joint consortium of Geophysical Laboratory at the Carnegie Institution of Washington and the APS) scientist Lin Wang, a research team from Carnegie Institution of Washington, Argonne, Stanford University, Jilin University, Xiangtan University, Stanford University, and the SLAC National Accelerator Laboratory examined yttrium oxide samples with grain sizes ranging from 5 nm to 1 μm, utilizing a variety of in situ high-pressure techniques, including x-ray diffraction, Raman spectroscopy, and high-energy pair distribution analysis at the HP-CAT 16-IDB and 16-BMD beamlines at the APS. After transmission electron microscopy revealed the same crystal structure in all of the samples under ambient conditions, the team subjected them to high pressures using diamond anvil cells with transmitting media of He and silicone oil.
The 16-nm, particle-sized material demonstrates increased stability compared to bulk Y2O3, with the cubic phase persisting up to 24.8 GPa (approximately 12.8 GPa greater than in the bulk material). As pressure is increased to above 24.8 GPa, disorder begins to appear, eventually yielding to a completely amorphous state above 30 GPa. The larger-grained 21-nm material, however, behaves considerably differently, maintaining its cubic structure up to 14 GPa, then undergoing a transition to the hexagonal phase up to 32.7 GPa. Meanwhile, micron-sized material transforms from cubic to the hexagonal phase at 12 GPa, which persists to the highest pressures measured. This demonstrates that yttrium oxide nanoparticles have a critical size above which they behave like the bulk Y2O3, but become amorphized below that size. Raman spectroscopy confirms the amorphized state of the samples subjected to high pressure.
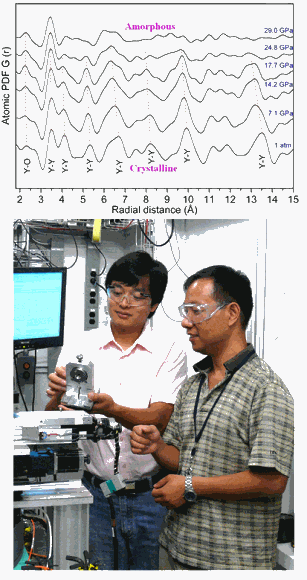
To further characterize this observed dependence of particle size on structural stability under pressure, high-energy pair distribution function (PDF) measurements were performed on 16-nm-sized samples. Using this technique, “We can identify differences of atom-to-atom linkage,” said co-author Wenge Yang of the Carnegie Institution. Because the distance between each pair of atoms in the structure can be determined by the position of peaks in the PDF data, shifts in those peaks represent changes in the atomic bonding length, while a complete disappearance of peaks indicates breakdown of the original linkage. Under increasing pressures, the connectivity of the YO6 octohedra in the cubic Y2O3 phase begins to break down, while the links between the edges shared by neighboring octahedra remain intact. Finally, at ~29 GPa and beyond, long-range ordering disappears and amorphization is completely dominant.
“This is the first time we’ve seen this size-dependent amorphization mechanism with the PDF measurement,” Yang said. The observed size effect explains the greater stability under pressure: While grain sizes of 21 nm and above transform from a cubic to hexagonal phase under pressure, the smaller-grained Y2O3 maintains the cubic phase for a longer period before amorphization sets in.
The research team plans to continue their work studying both Y2O3 and other materials, examining how various nanomaterial and bulk compounds with differing particle sizes behave under extreme conditions. Their findings will have direct implications regarding the manufacture and versatility of nanomaterials for various applications. As the current work demonstrates, the high-energy PDF measurement technique adds an important new tool to the experimental toolbox. “We’ve opened up a new way to look at the disordering mechanism of nanomaterials,” said Yang. — Mark Wolverton
See: LinWang1,2*, Wenge Yang1, Yang Ding1, Yang Ren3, Siguo Xiao4, Bingbing Liu2, Stanislav V. Sinogeikin1, Yue Meng1, David J. Gosztola3, Guoyin Shen1, Russell J. Hemley1, Wendy L. Mao5,6, and Ho-kwang Mao1, “Size-Dependent Amorphization of Nanoscale Y2O3 at High Pressure," Phys. Rev. Lett. 105, 095701 (27 August 2010).
DOI: 10.1103/PhysRevLett.105.095701
Author affiliations: 1Carnegie Institution of Washington, 2Jilin University, 3Argonne National Laboratory, 4Xiangtan University, 5Stanford University, 6SLAC National Accelerator Laboratory
Correspondence: *wanglin@aps.anl.gov
The research was supported by NSF (MRI-0821584 and EAR-0810255), and the International Balzan Foundation. HPSynC is supported as part of EFree, an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences (BES) under Grant Number DE-SC0001057. HPCAT is supported by CIW, CDAC, UNLV and LLNL through funding from DOENNSA, DOE-BES and NSF. The work was conducted at the APS, the Electron Microscopy Center for Materials Research, and the Center for Nanoscale Materials at Argonne under Contract No. DE-AC02-06CH11357). This work was also partially supported by the NSFC (10979001), the National Basic Research Program of China (2005CB724400).
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science, Office of Basic Energy Sciences (DOE-BES). The APS is the source of the Western Hemisphere’s brightest high-energy x-ray beams for research in virtually every scientific discipline. More than 3,500 scientists representing universities, industry, and academic institutions from every U.S. state and several foreign nations visit the APS each year to carry out applied and basic research in support of the BES mission to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels in order to provide the foundations for new energy technologies and to support DOE missions in energy, environment, and national security. To learn more about the Office of Basic Energy Sciences and its x-ray user facilities.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.