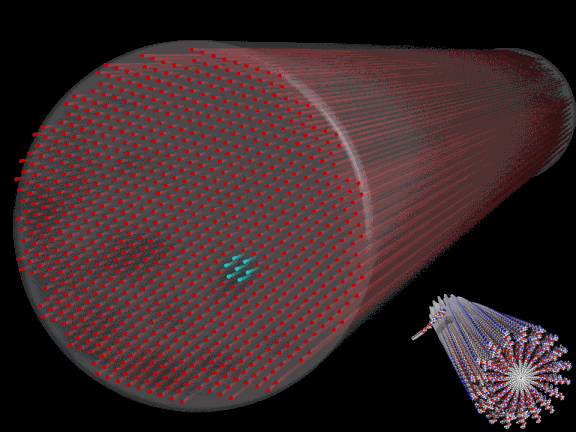
Experiments can sometimes lead to the discovery of completely unanticipated phenomena. Such is the case with the remarkable behavior exhibited by peptide nanostructures (in the form of supramolecular filaments) observed during experiments carried out by researchers from Northwestern University at beamline 5-ID of the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) Synchrotron Research Center at the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne National Laboratory.
According to Professor Samuel Stupp, lead author of the study recently published in Science, while “trying to elucidate the hierarchical organization of peptide nanostructures” his team discovered that when dispersed in water, those filamentary nanostructures could organize into hexagonally-packed bundles. The researchers were surprised to find that at sufficiently high concentrations in solution, the filaments could spontaneously self-assemble into crystalline structures (the hexagonally-packed bundles). Even more surprising was the finding that the x-rays used to probe the nanostructures also sometimes triggered filament crystallization. This work may impact our understanding of nanostructures in biological systems and our ability to control the structure of materials.
The filaments used for this research possessed diameters of around 10 nanometers and lengths on the order of tens of micrometers. The filaments were derived from a synthetic molecule containing a short peptide sequence. Peptides are compounds containing two or more amino acids. Here, the peptide sequence consisted of six alanine amino acid molecules bonded to three glutamic acid molecules – abbreviated Ala6Glu3 – which in turn was grafted to an alkyl molecule. The resulting “supramolecules” self-assembled in water to form the filaments.
A sequence of experiments was designed to reveal the arrangement of the filaments dispersed in water. Different aqueous concentrations of the filaments were placed inside tiny 2-mm-diameter quartz capillaries and studied utilizing small-angle x-ray scattering (SAXS) at the DND-CAT beamline. The concentrations ranged from 0.5 to 5 weight percent. The SAXS data revealed that all concentrations of filaments aggregated into bundles exhibiting a hexagonal packing (see Fig. 1). The organization of the filaments into hexagonally-packed bundles (i.e., crystallization) is quite remarkable. But even more remarkable was the observation that the higher concentration of filaments (2 and 5 weight percent) spontaneously crystallized, while the lower-concentration solutions (0.5 and 1 weight percent) crystallized only through x-ray exposure.
According to Prof. Stupp, the crystallization of the filaments, either by self-assembly or by x-ray exposure, constitute phenomena that “we have not seen before” in other supramolecular systems. Stupp also observed that “in doing the experiments at the APS synchrotron, we were surprised to find that x-rays could promote crystallization.”
A fascinating feature of the x-ray-induced crystallization was the reversibility of the process, which was actually visible. Using the 1 weight percent solution, a cumulative 200 seconds of x-ray irradiation turned the initially-transparent solution opaque, indicating crystallization. After x-ray cessation, the solution’s opacity slowly decreased until it was clear again within about 40 minutes, indicating a return to disorder. A follow-up SAXS experiment exposed the solution to a number of 4-second x-ray bursts. The experimental data showed that the initially-unordered filaments (revealed by the first 4-second exposure) gradually underwent a change to hexagonally-ordered bundles of filaments as recorded during the last x-ray exposures. When the experiment was repeated two hours later, the SAXS data revealed the filaments were once again disordered – the crystalline structure had disappeared.
The researchers considered whether extraneous factors might have contributed to filament ordering. Intense x-rays can create new chemical compounds within a solution due to ionization, as well as produce subtle heating. However, subsequent tests of the filamentary solutions showed that neither unwanted chemical species, nor thermal effects, had played a part in either the spontaneous or x-ray-triggered crystallizations.
Concerning the basic mechanism responsible for crystallization, the researchers envision that the long-term stability of the crystalline domains is a balance between two opposing tensions: electric charges residing on the filaments (either native or induced by x-ray irradiation) tend to push filamentary bundles apart, while entrapment of filaments within the larger network leads to an inward mechanical compression.
Experimental data revealed that as filament concentration grew, the number of filaments within bundles increased as well, until a critical concentration of filaments resulted in their spontaneous hexagonal arrangement within the bundles (i.e. crystallization). On the other hand, lower filamentary concentrations – unable to spontaneously crystallize – could only do so when x-rays increased the charge density on the filaments’ surfaces, thereby changing the balance of inter-filament forces in favor of crystallization.
The same mechanism that created their man-made crystalline filamentary networks may well be at work in biological cells, leading Prof. Stupp to observe that “this research could help us understand the organization of nanostructures in biological systems, and may also have applications in controlling the structure of materials.” — Philip Koth
See: Honggang Cui, E. Thomas Pashuck, Yuri S. Velichko, Steven J. Weigand, Andrew G. Cheetham, Christina J. Newcomb, and Samuel I. Stupp*, “Spontaneous and X-ray–Triggered Crystallization at Long Range in Self-Assembling Filament Networks,” Science 327, 555 (29 January 2010). DOI: 10.1126/science.1182340
Author affiliation: Northwestern University
Correspondence: *s-stupp@northwestern.edu
This work was supported by the U.S. Department of Energy (Grant No. DE-FG02-00ER45810). The DND-CAT located is supported by E. I. DuPont de Nemours and Company, Dow Chemical Company, and the State of Illinois. Use of the APS was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. Some additional support for this work was obtained from National Institutes of Health National Institute of Dental and Craniofacial Research grant 5R01 DE015920 and National Science Foundation grant DMR-0605427.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.