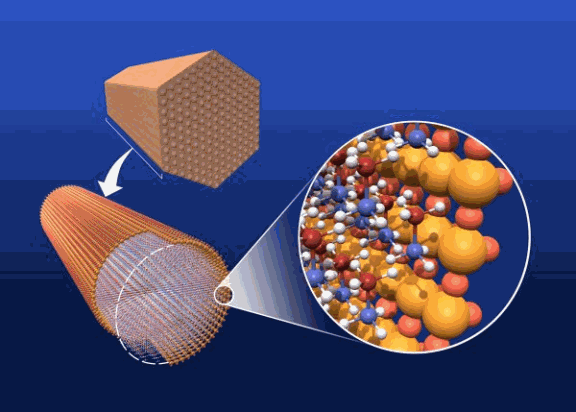
When it comes to squeezing hydrogen out of ammonia borane the packaging matters, according to scientists from three U.S. Department of Energy national labs. Ammonia borane releases hydrogen with heating by a multi-step reaction, but the nominal heating required to release that hydrogen requires additional energy, decreasing the overall efficiency. The scientists found that less heat is needed to free the hydrogen when the ammonia borane is confined in a mesoporous form of silica.
In a recent study, they demonstrated the power of a relatively new method called "atomic pair distribution function" to study how mesoporous materials influence the molecules they confine. This new method provides far more insight into the nature of nanophase materials than conventional techniques.
This study was done by an interdisciplinary team from Los Alamos National Laboratory, Pacific Northwest National Laboratory (PNNL), and Argonne National Laboratory.
Remote outposts, such as telecommunications towers, or emergency back-up power in isolated locations could benefit from reliable long lasting power supplies. While conventional methods are cumbersome or severely limited, hydrogen or other chemical bonds coupled with fuel cells could provide the needed energy. One of the stumbling blocks to making this happen is understanding, and ultimately controlling, the reactions that release and store the hydrogen.
The team began with synchrotron x-ray powder diffraction experiments on ammonia borane or NH3BH3 embedded in a form of mesoporous silica known as MCM-41. The silica contained pockets measuring between 2 and 50 nanometers across. The scientists obtained diffraction patterns for ammonia borane across a temperature range from 80K to 300K, which translates to about -315 to 80° Fahrenheit.
The x-ray powder diffraction experiments were done at the XOR/BESSRC 11-ID-B beamline at the Advanced Photon Source at Argonne National Laboratory.
Next, the team used an analytical technique known as atomic pair distribution function or PDF to unravel the x-ray data. "This type of analysis tells you about the nanoscale ordering of the material," said Dr. Tom Autrey, a Pacific Northwest National Laboratory scientist on the team. "This analysis lets you look at atom pairs and understand group interactions."
The researchers found that ammonia borane changed from a well-ordered molecular crystal to a disordered solid at temperatures around 270K (26.33°F). Further, the ammonia borane skipped a structural change that the unconfined molecules experience at 225K (54° F).
The results of the atomic pair distribution analysis, combined with their earlier studies, suggested that a mesoporous silica packaging could play a key role in allowing hydrogen-rich molecules to release hydrogen at lower temperatures, costing less energy to get the reaction going, and providing hydrogen with fewer impurities.
Autrey and his colleagues are looking at using mesoporous silica and similar materials to develop new approaches for the activation of molecular hydrogen. The activated hydrogen could be used to produce higher quality biofuels or be used to convert carbon dioxide to renewable fuels without the use of expensive metal catalysis.
"Catalysis is critical to a whole range of different energy applications, but catalytic activation of hydrogen without metals, well, that can be a game changer," said Autrey.
The original PNNL press release can be found here.
See: H. Kim1*, A. Karkamkar2, T. Autrey2*, P. Chupas3, and T. Proffen1, "Determination of Structure and Phase Transition of Light Element Nanocomposites in Mesoporous Silica: Case Study of NH3BH3 in MCM-41," J. Am. Chem. Soc. 131(38),13749 (20009). DOI: 1021/ja904901d
Author affiliations: 1Los Alamos National Laboratory, 2Pacific Northwest National Laboratory, 3Argonne National Laboratory
Correspondence: *hjkim@lanl.gov; **tom.autrey@pnl.gov
Work performed at Argonne National Laboratory and use of the Advanced Photon Source was supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. H.K. and T.P. acknowledge support from the Lujan Neutron Scattering Center, funded by the DOE Office of Basic Energy Sciences. Los Alamos National Laboratory is operated by Los Alamos National Security LLC under Contract DE-AC52-06NA25396. T.A. and A.K. acknowledge support from the U.S. Department of Energy, Office of Basic Energy, Division of Chemical Sciences, Biosciences and Geosciences. Pacific Northwest National Laboratory is operated by Battelle for the U.S. Department of Energy.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.