In today's world of sophisticated organisms, proteins are the stars. They are the indispensible catalytic workhorses, carrying out the processes essential to life. But long, long ago ribonucleic acid (RNA) reigned supreme. Now researchers from Northwestern University and The University of Chicago, using the Life Sciences Collaborative Access Team x-ray research facility at the U.S. Department of Energy’s Advanced Photon Source, have produced an atomic picture that shows how two of these very old molecules interact with each other. It is a rare glimpse of the transition from an ancient, RNA-based world to our present, protein-catalyst dominated world.
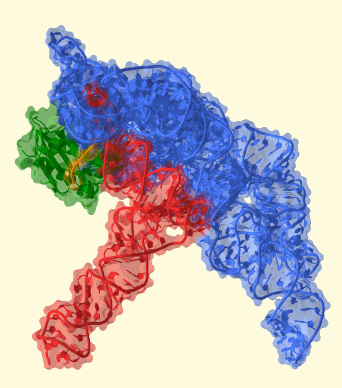
These researchers are the first to show the atomic details of how ribonuclease P (RNase P) recognizes, binds, and cleaves transfer RNA (tRNA). They used the powerful x-rays produced by the Advanced Photon Source at Argonne National Laboratory to obtain images from crystals formed by these two RNA molecules. The result is a snapshot of one of the most complex models of a catalytic RNA and its target.
“RNA is an ancient molecule, but it is pretty sophisticated,’ said Alfonso Mondragón, Professor of Molecular Biosciences in the Weinberg College of Arts and Sciences at Northwestern, who led the research team. “Our crystal structure shows that it has many of the properties we ascribe to modern molecules. RNA is a catalyst that has much of the versatility and complexity of modern-day proteins.”
For billions of years, and still to this day, the function of RNase P, which is found in nearly all organisms, from bacteria to humans, has been to cleave transfer tRNA. If the tRNA is not cleaved, it is not useful to the cell.
“We knew this important chemistry happened, that RNA acts as a catalyst, but we didn't know exactly how until now,” Mondragón said. “We now have a better understanding of how RNA works.”
RNase P is formed by a large RNA core plus a small protein, illustrating the evolutionary shift from an RNA world toward a protein-dominated world. The protein helps recognize the tRNA, but most of the recognition occurs through RNA-RNA interactions involving shape complementarity and also base pairing.
The structure shows that once RNase P recognizes tRNA, it docks and, assisted by metal ions, cuts one chemical bond. This matures the tRNA, producing a smaller RNA molecule that now can contribute to fundamental processes in the cell. The RNA-based enzyme does this over and over, cutting each tRNA in exactly the same place every time.
“The discovery nearly 30 years ago that RNA molecules can have a catalytic function raised the idea that maybe RNA was the first molecule,” Mondragón said. “Our work reinforces this notion of the existence of an RNA world when life first began.”
See: Nicholas J. Reiter1, Amy Osterman1, Alfredo Torres-Larios1‡, Kerren K. Swinger1‡ ‡, Tao Pan2, and Alfonso Mondragón1, “Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA,” Nature, Published online 14 November 2010. DOI:10.1038/nature09516
Author affiliations: 1Northwestern University, 2The University of Chicago Present addresses: ‡Universidad Nacional Autónoma de México, ‡‡Abbott Laboratories
Correspondence: a-mondragon@northwestern.edu
This research was supported by the National Institutes of Health. N.J.R. is a National Research Service Award postdoctoral fellow. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
The original Northwestern University press release by Megan Fellman can be found here.
The Advanced Photon Source is an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory. The APS is the source of the Western Hemisphere’s brightest high-energy x-ray beams for research in virtually every scientific discipline. More than 3,500 scientists representing universities, industry, and academic institutions from every U.S. state and several foreign nations visit the APS each year to carry out applied and basic research in support of the DOE mission to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels in order to provide the foundations for new energy technologies and to support DOE missions in energy, environment, and national security. To learn more about DOE x-ray user facilities.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.