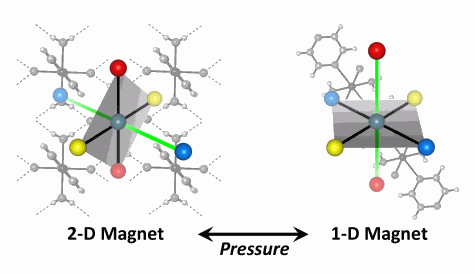
A material’s properties are a critical factor in the way that material can be used for practical applications. Magnetism is one such property, and magnetic switches are key components for advances in data storage and for displays in computers and cell phones. These properties are normally adjusted (or “tuned”) by changing the “recipe” during preparation. Now scientists at the U.S. Department of Energy’s (DOE’s) Argonne National Laboratory, along with a collaborator from Eastern Washington University, have harnessed the power of extreme high pressure and discovered a novel approach to predictably tune the switching of a promising new family of next-generation magnetic materials. Their results were published in the online version of Angewandte Chemie International Edition on September 10, 2010.
The magnetic materials the team studied are made from copper ions and simple building blocks, including water and fluoride. Their simple structures are held together by strong hydrogen bonds, making very robust molecular networks. Each copper ion, which sits at the corner of a molecular cube, contains one unpaired electron. These spins are disordered at normal temperatures, but begin to align in opposite directions at low temperatures, creating the magnetic state called antiferromagnetism.
Using state-of-the-art high-pressure equipment and the high-energy, highly focused x-ray beams from the X-ray Science Division 1-BM beamline at the Argonne Advanced Photon Source, the scientists observed a series of structural transitions as they exerted pressure on the material. These rearrangements abruptly reoriented the magnetic spins of the material, creating a reversible magnetic switch effect.
High-pressure science has traditionally been the domain of Earth scientists and, until now, has not been routinely used to study molecular systems. Such studies promise to greatly improve our understanding of the often complex way materials’ functional properties are related to their molecular structures—a key step in realizing the diverse potential of molecular materials.
“Molecule-based materials are much softer than traditional solid-state materials, like oxides and minerals,” said Argonne scientist Gregory Halder. “As such, we can expect to induce dramatic changes to their structures and functional properties at relatively low, industrially relevant pressures.”
Next up for this team of researchers: Studying a targeted series of molecular network materials under pressure to understand the broader implications of this new phenomenon.
See: Gregory J. Halder1*, Karena W. Chapman1, John A. Schlueter1, and Jamie L. Manson2, “Pressure-Induced Sequential Orbital Reorientation in a Magnetic Framework Material,” Angew. Chem. Int. Ed. 49, (2010). (Published online September10, 2010). DOI:10.1002/anie.20100
Author affiliations: 1Argonne National Laboratory, 2Eastern Washington University
Correspondence: *halder@aps.anl.gov
Work performed at Argonne National Laboratory and use of the Advanced Photon Source were supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. Part of the pressure cell preparation used the GSECARS facility (Sector 13), Advanced Photon Source, Argonne National Laboratory. GSECARS is supported by the NSF-Earth Sciences (EAR-0622171) and DOE-Geosciences (DE-FG02-94ER14466). Work at EWU was partially supported by the National Science Foundation under Grant No. DMR-1005825.
The Advanced Photon Source is an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory. The APS is the source of the Western Hemisphere’s brightest high-energy x-ray beams for research in virtually every scientific discipline. More than 3,500 scientists representing universities, industry, and academic institutions from every U.S. state and several foreign nations visit the APS each year to carry out applied and basic research in support of the DOE mission to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels in order to provide the foundations for new energy technologies and to support DOE missions in energy, environment, and national security. To learn more about DOE x-ray user facilities.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.