Portland cement concrete is all around us, in the sidewalks we walk on, the buildings we live and work in, and the roads our vehicles ride on. One of the most versatile and useful building materials ever created, it is so commonplace that we hardly ever think of it (unless we happen to trip and scrape our knees on it). Yet surprisingly, although we worry about carbon emissions from cars, airplanes, and power plants, this everyday, seemingly innocent material is also a hidden and largely ignored global warming culprit. Now, new insights into the nanostructure of concrete are coming to light thanks to studies carried out at the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne National Laboratory. The work, by researchers from the University of California, Berkeley, and Argonne is an important milestone in the push for stronger, more environmentally friendly concrete.
“The manufacture of cement is responsible for about 5% to 7% of all the CO2 released by humans into the Earth’s atmosphere every year,” said Paulo Monteiro of the University of California, Berkeley, lead author of the paper published in Physical Review Letters. “We know that it is quite possible that the federal government will start putting charges on the use of concrete to account for its carbon footprint. The cement industry is already kind of bracing for it.” Such a “carbon tax” would inevitably lead to higher costs for concrete.
Those sobering facts have set the cement and concrete industry on an intensive quest to find ways to reduce CO2emissions while improving concrete’s strength and versatility, qualities which are still not completely understood, even though Portland cement has been around for well over a century. A better understanding of the atomic-level structure of cement hydration products is the key to finding more environmentally-friendly ways to manufacture and use concrete.
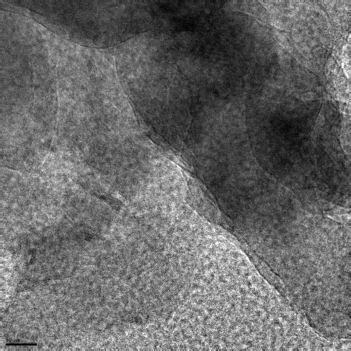
The researchers from the University of California and Argonne studied the nanostructure of calcium silicate hydrate (CSH), the main ingredient that gives concrete its great strength, “The glue that holds concrete together,” as Monteiro put it. Pinning down the crystalline structure of CSH in cement-paste matrix has proven elusive because of its broad diffraction properties and difficulty in separating it from the other complex phases of cement formation.
“In previous times, people would refer to these calcium silicates as almost like an amorphous gel, indicating there was very little structure in the very short range,” said Monteiro. Only recently have more precise characterization techniques become available to probe into the details of CSH structure. Using synchrotron x-ray diffraction at the X-ray Science Division 11-ID-C beamline of the APS, Monteiro and his colleagues found that CSHs are more ordered than previously believed, and at a smaller scale.
The bulk behavior of CSHs has been explained theoretically by some colloidal models that posit the existence of nanograins of 5 nm or less, but experimental confirmation has been lacking because of the previously-mentioned diffraction and phase separation problems. Crystallographic work has also suggested a strong similarity to the rare toberomite minerals, but in a less structured form. To better investigate these possibilities and refine the models, the research team used pair distribution function techniques on samples of a synthetic CSH(1) and a hydrated tricalcium silicate paste (hyd-C3S). Each sample represents different phases of CSHs as they are found in real cement paste.
The experimenters found a lack of sharp Bragg peaks in the synthetic CSH(1) compared to the hyd-C3S, indicating that while the CSH is not truly amorphous, it also contains no large, ordered crystals. However, it shows striking similarity to a size-broadened 1.1-nm toberomite crystal structure. Damping of structural features is seen at scales larger than 3.5 nm, confirming a nanocrystalline structure of the CSH with an approximately 3.5-nm particle diameter. This supports previously published work, including small-angle neutron scattering measurements that implied a similar nanostructure. “Even using completely different techniques with x-rays, we did get complementary information,” Monteiro said. “That’s good validation, but the unique contribution of our work is that the nanocrystals are in fact very ordered and that the planes need only be slightly bent to inhibit growth.”
The next step will be to investigate the possible role of polymers and other materials—possibly including fly-ash residue from coal—that could be used to make hybrid CSHs with improved cementitious properties and a reduced carbon footprint. These new insights into the nanostructure of concrete are an important milestone in optimizing the engineering of improved CSHs for creating stronger, more environmentally benign concrete. — Mark Wolverton
See: L.B. Skinner1, S.R. Chae1, C.J. Benmore2, H.R. Wenk1, and P.J.M. Monteiro1*, “Nanostructure of Calcium Silicate Hydrates in Cements,” Phys. Rev. Lett. 104(19), 195502 (2010). DOI:10.1103/PhysRevLett.104.195502
Author affiliations: 1University of California, Berkeley; 2Argonne National Laboratory
Correspondence: *monteiro@ce.berkeley.edu
See also: Physical Review Focus: “The Nanostructure of Buildings and Bridges,” by Michelangelo D'Agostino, 7 May 2010.
This publication was based on work supported in part by Grant No. KUS-l1-004021,made by King Abdullah University of Science and Technology (KAUST). This work and use of the Advanced Photon Source at Argonne National Laboratory was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.