Ever since the Bronze Age, humans have experimented with combining different metals to create alloys having properties superior to either metal alone. But not all metals readily form alloys. For some pairs of elements the atoms are too dissimilar. Now, researchers in an international team, using high-brilliance x-rays from the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne, have discovered that previously impossible alloys can be created by subjecting atoms to high pressure―opening possibilities for new materials in the future.
Previous studies have shown that at pressures thousands of times larger than atmospheric pressure, atoms change their properties and combine under different rules, creating new materials. But the cerium-aluminum alloy produced at the Carnegie Institution’s Geophysical Laboratory as an outgrowth of research at the APS breaks new ground in the development of alloys.
“The boundary has been pushed before, but not to this extreme,” said Carnegie’s Ho-kwang Mao, who with Jian-zhong Jiang of the International Center for New-Structured Materials, Zhejiang University, China, led the research team which also included scientists from Stanford University and Uppsala University, Sweden. Their results are published in the Proceedings of the National Academy of Sciences.
Most alloys are “substitutional alloys,” meaning that the atoms of the two metals are randomly intermingled within a single atomic structure. But this can only happen if the two types of atoms are approximately the same size and have nearly the same electronegativity (a propensity to attract electrons). By these criteria, known as the Hume-Rothery rules, cerium and aluminum are incompatible. Both cerium and aluminum form many useful alloys with other metals. But cerium atoms are 28% bigger than aluminum atoms, and have a significantly lower electronegativity. The two elements can form chemical compounds together, as well as disordered mixtures called metallic glasses, but a cerium-aluminum alloy appeared to be off limits.
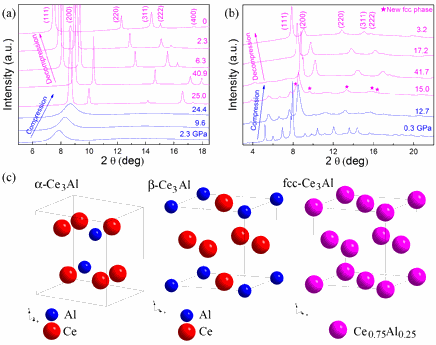
The team carried out high-pressure experiments at the High Pressure Collaborative Access Team beamline 16-ID-B and the X-ray Operations and Research/PNC 20-BM-B beamline, both at the APS. When a sample of cerium-aluminum metallic glass began to show the properties of an alloy, the research team took notice. At 25 gigapascals (approximately 250,000 atmospheres) of pressure, the mismatched atoms overcame their differences and settled into a single crystal structure. “It wasn’t totally unexpected,” said Mao. “Usually when you apply pressure in a case like this the bigger atom becomes smaller, but normally by just a few percent, not enough to allow an alloy to form.”
In the case of cerium, however, pressure causes the atoms to shed some of their outermost electrons, making the electrons “delocalized.” The delocalization of the electrons not only changes the electronegativity of the cerium atoms, it causes the atoms to collapse in volume by 15%. The net result is that both size and electronic structure are put in a range where the cerium and aluminum atoms can comfortably nestle together, forming an alloy.
“This is a very interesting result, in which two very different atoms can have the difference erased by the pressure effect,” said Mao. “The second interesting thing is that it’s quenchable, meaning that when we release the pressure, the alloy persists. A lot of high-pressure materials can be made, but once the pressure is reduced they go back to their original form. You cannot make anything useful from those materials. But this alloy is quenchable, so that creates possibilities.”
The researchers are currently investigating the properties of the new alloy. One key finding is that after quenching, the delocalized electrons become localized again, suggesting that the alloy may retain some of cerium’s magnetic properties. Rare-earth elements such as cerium are components of the strongest known magnets. The new alloy could also have novel electronic and mechanical properties.
Mao notes that the success in producing this new alloy implies that other potentially useful combinations can be made under high pressure. “Aluminum is an archetypal material which has small atoms and forms many alloys with other elements with small atoms,” he says. “These other elements, such as magnesium, can probably also form alloys with cerium. So this result will open a lot of doors for new materials to study.”
See: Qiao-Shi Zeng, Yang Ding, Wendy L. Mao, Wei Luo, Andreas Blomqvist, Rajeev Ahuja, Wenge Yang, Jinfu Shu, Stas V. Sinogeikin, Yue Meng, Dale L. Brewe, Jian-Zhong Jiang*, and Ho-kwang Mao**, “Substitutional alloy of Ce and Al,” Proc. Nat. Acad. Sci. 106(8), 2515 (February 24, 2009) . DOI: 10.1073_pnas.0813328106
Contacts: *jiangjz@zju.edu.cn, **hmao@gl.ciw.edu@gl.ciw.edu
Use of the High Pressure Collaborative Access Team facility was supported by the Department of Energy, Office of Basic Energy Sciences (DOE-BES); the Department of Energy, National Nuclear Security Administration (Carnegie/Department of Energy Alliance Center); the National Science Foundation; the Department of Defense, Tank, Automotive and Armaments Command; and the W.M. Keck Foundation. X-ray Operations and Research/PNC facilities at the APS, and research at these facilities, are supported by the DOE-BES, a major facilities access grant from Natural Sciences and Engineering Research Council of Canada, the University of Washington, Simon Fraser University, and the APS. This work was supported by the Balzan Foundation; National Natural Science Foundation of China Grants 50425102, 50601021, 50701038, 60776014, 60876002, and 10804096;the Zhejiang University–Helmholtz Cooperation Fund; the Ministry of Education of China (Program for Changjiang Scholars, the Research Fund for the Doctoral Program of Higher Education from China Scholarship Council); the Department of Science and Technology of Zhejiang Province; and Zhejiang University. W.L., A.B., and R.A. are grateful to the Swedish Research Council for providing financial support, the Swedish National Infrastructure for Computing, and the Uppsala Multidisciplinary Center for Advanced Computational Science for providing computational resources.
Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America 's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.