Biologists using an x-ray beamline at the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne have worked out a rudimentary architectural plan for the nuclear pore complex (NPC), the gatekeeper of the cell's nucleus. Their finding reveals a remarkable evolutionary story dating back more than one billion years.
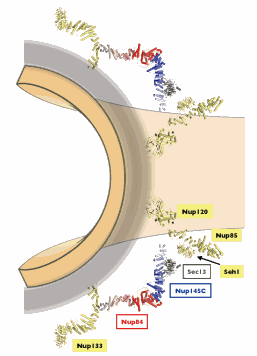
The massive NPC, which allows molecules such as RNAs and proteins to move in and out of the nucleus, is one of the largest macromolecular structures in the cell (containing about 5 million atoms). Its structure has been considered a "holy grail" in structural biology for many decades. The researchers from the Massachusetts Institute of Technology (MIT) found that the NPC scaffold forms an open lattice structure, similar to another membrane coating complex, called COPII. That complex has far fewer components and is involved in a completely different cellular process, called vesicular transport, by which materials move in, out and around the cell enclosed in vesicles.
The finding reveals a remarkable evolutionary story: The proteins that form the building blocks in the NPC and the COPII vesicle coats are so unique that they still have not been identified anywhere else in the cell. Once the models of the proteins were complete, it became clear that these two cellular structures share a common ancestor, dating back over one billion years, which already contained the same building blocks connected in very similar fashion. Second, discovering the specific relationship between the NPC and the much simpler, better understood COPII vesicle coat constitutes a significant step toward understanding the entire NPC assembly, which appears to be remarkably modular and most likely self-assembling.
MIT graduate student Stephen Brohawn created a complex of three nuclear pore proteins in bacteria and grew microscopic crystals of the complex. Then he solved the structure of the complex by x-ray crystallography using the Northeastern Collaborative Access Team 24-ID-C/E beamline at the APS and beamline X29 at the National Synchrotron Light Source at Brookhaven National Laboratory. In general, complexes as large as this one—especially nuclear pore proteins—are notoriously difficult to crystallize in order to solve their structure, but strategies such as solving the structure of small pieces of the complex separately made the problem tractable.
Understanding structurally that the NPC and the COPII vesicle coat are evolutionarily related opens a wide avenue of future research. The structures now available are still only pieces within the entire nuclear pore complex puzzle. However, unraveling the common architecture serves as a guide to the next steps toward understanding the entire transport machine. Also, several other ideas about the NPC architecture that were largely speculative can now be put to rest.
"A complete structure of the NPC is the project of a lifetime, but we have shown that it is feasible and worth it, and that many unexpected discoveries can be made on the road as well," said Schwartz.
See: Stephen G. Brohawn and Thomas U. Schwartz*, "Molecular architecture of the Nup84-Nup145C-Sec13 edge element in the nuclear pore complex lattice," Nature Structural & Molecular Biology, published online 25 October 2009. DOI: 10.1038/nsmb.1713
Author affiliation: Massachusetts Institute of Technology
Correspondence: * tus@mit.edu
Visit The Schwartz Lab Web site at http://schwartzlab.mit.edu/
This work was supported by National Institutes of Health grant GM77537, a Pew Scholar award (to T.U.S.), and a Koch Fellowship (to S.G.B). Brookhaven National Laboratory is funded by the U.S. Department of Energy's Office of Science.
Use of the Advanced Photon Source is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.