Porous materials frameworks with open structures, containing substantial numbers of cavities and extended internal surfaces where smaller guest molecules can attach, are potentially useful for a wide range of applications, such as gas storage, catalysis, and drug delivery. One class of such open-framework materials is metal-organic frameworks (MOFs), with metal ions or clusters of ions fixed in a scaffold of organic linkers. The more porous these frameworks are, with bigger open spaces, the more fragile they tend to become. Now, researchers have made a MOF that is remarkably open but also stable through the incorporation of cavities that are larger than 20 Å (mesocavities) but with openings less than 20 Å (microwindows), a strategy demonstrated by x-ray diffraction studies at the ChemMatCARS beamline 15-ID-B at the U.S. Department of Energy’s Advanced Photon Source at Argonne National Laboratory. This new synthetic strategy may serve as a general approach toward stable MOFs with even higher surface areas, eventually leading to even greater practical applications.
A standard way to construct MOFs is to connect metal ions using organic linking molecules, or ligands. Solvothermal reactions, which are carried out in organic solvents under high temperature, are used to form bonds between the linkers and the metal ions. Increasing the size of the ligands will expose otherwise hidden edges of these molecules, leading to higher surface area. However, the frameworks tend to be “fragile,” reducing their practical value.
Researchers at Texas A&M University and Shandong University in China chose as their building blocks copper-containing cuboctahedra, well-known structures that have 6 square faces and 8 triangular faces enclosing a cavity about 13 Å (1.3 nm) across. To incorporate the cuboctahedra within MOFs in a novel way, Hong-Cai Zhou of Texas A&M and his colleagues designed two new ligands, each one a planar molecule with three arms extending out at 120° from each other. The ligands are similar in molecular form, with one having slightly longer arms than the other.
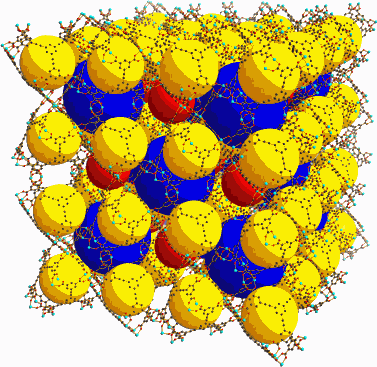
Solvothermal reactions of the ligands with copper salts formed a three-dimensional arrangement of cuboctahedra, with a well-defined crystal structure, that the researchers call a porous coordinated network (PCN). Use of the smaller ligand produces a structure in which the cuboctahedra pack together leading to cavities of two distinct sizes arranged in a regular way. X-ray powder diffraction of this crystalline solid, dubbed PCN-61, reveals that the smaller of the two cavities (yellow in the accompanying diagram) are 15 Å across, while the larger are 23 Å. PCN-66, the metal-organic framework formed using the larger ligand has the same cuboctahedra packed similarly, leading to slightly larger cavities, measuring 16 Å and 26 Å, respectively.
X-ray diffraction measurements also show that the PCN structures do not change when small molecules adsorbed in their interior cavities are removed. That is in contrast to some other large-pored metal-organic frameworks, whose cavities have been found to collapse when they are empty. The stability of the large mesoscale cavities in the PCNs comes about, Zhou says, because their surfaces are formed from the rigid ligands and the relatively small faces of the cuboctahedra, which control the size of the openings of the cavities and give stability to the overall structure.
The cuboctahedra faces act as “microwindows” opening to the interior of the mesocavities. Zhou likens the PCN structure to a building that has many large rooms with small doors and narrow hallways connecting them. A large number of people could fit into the rooms, he explains, but the small doors control how fast they could move in and out. Similarly, the PCNs can store large amounts of gas inside the cavities, but the rate of gas molecules going in and out is controlled by the microwindows.
These porous molecules have large internal surfaces on which smaller molecules may be adsorbed. For PCN-61, the surface area amounts to 3,000 to 3,500 m2 per gram, depending on how it is estimated, while for PCN-66, constructed with the larger ligands, the surface area is from 4,000 to 4,600 m2 per gram -- among the highest values ever found for metal-organic frameworks. Zhou and his colleagues are now working to employ the techniques demonstrated with these two frameworks to build framework structures with still larger pores and surface areas. — David Lindley
Correspondence: *zhou@mail.chem.tamu.edu
See: Dan Zhao1, Daqiang Yuan1, Daofeng Sun2, and Hong-Cai Zhou1*, "Stabilization of Metal-Organic Frameworks with High Surface Areas by the Incorporation of Mesocavities with Microwindows," J. Am. Chem. Soc. 131, 9186 (2009). DOI: 10.1021/ja901109t
Author Affiliations: 1Texas A&M University; 2Shandong University
This work was supported by the U.S. Department of Energy (DE-FC36-07GO17033), the U.S. Defense Logistics Agency (N00164-07-P-1300), and the U.S. National Science Foundation (CHE-0449634). The microcrystal diffraction was carried out with the kind assistance of Yu-Sheng Chen at Argonne National Laboratory (CHE-0535644, DEAC02-06CH11357).
Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences (DOE-BES), under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America 's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.