Because the atomic structure and polarity of ferroelectric materials respond dramatically to an applied electric field, they have found increasing application in such areas as electromechanical actuators, nonvolatile computer memories, and advanced radio-frequency electronics. But what if there were another way to make ferroelectric materials do their thing—not electrically by applying a voltage, but through another mechanism? A group of experimenters from Argonne National Laboratory, the University of Pennsylvania, and Northern Illinois University has managed to do just that, proving that not just electricity but also a little bit of chemistry can flip the structure and thus the polarity of a ferroelectric film.
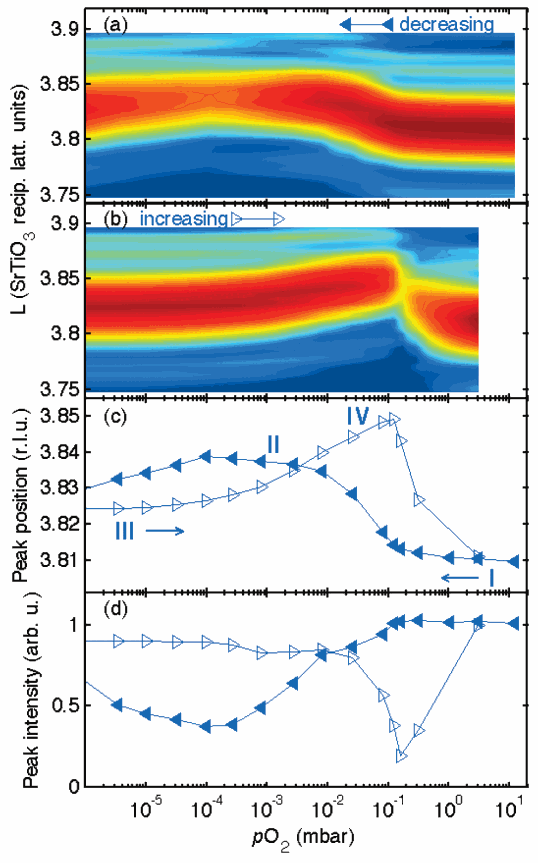
The researchers studied very thin lead titanate (PbTiO3) films, 2 to 21 nm in thickness, grown on SrRuO3 films on SrTiO3substrates. While varying the oxygen partial pressure (pO2) on the top surface of the lead titanate film at temperatures from 550K to 950K, the team used synchrotron x-ray scattering at the X-ray Operations and Research/BESSRC 12-1D-D beamline at the U.S. Department of Energy’s Advanced Photon Source at Argonne National Laboratory. The studies were carried out at 28.3 keV in order to monitor the structural changes in the film. Team member Brian Stephenson, of the Argonne Materials Science Division, explained, “We use the x-rays to see the structure of the film so we can watch this in situ while it’s happening. There actually aren’t that many ways to really see what’s going on in these thin films.” The x-ray technique, however, “gives us more direct information than people have had before.” As the pO2 was altered, hysteresis was evident in the film structure, indicating polarization changes. “All of the unit cells in the lead titanate change their structure when the surface is exposed to a different oxygen pressure,” said Stephenson. “At high oxygen pressure, the polarization points up, and if you go to low oxygen pressure, you can switch it and make it point down. Furthermore, the switching back and forth can be repeated in both directions many times without permanent change; it is thermodynamically reversible. ” During the polarization switching process, scattering interference patterns show that domains of both directions are present.
The team’s finding, that the chemically-induced polarization switching could be reproduced and repeated under control is quite significant, because it indicates that the chemical environment in which these ultrathin ferroelectrics operate is more important and influential than previously believed. It is a phenomenon that needs to be taken into account as these materials are created and used. As Stephenson pointed out, “Not only are the electronic properties of the interface important, but [so is] the chemistry: whether there are extra ions of one sign or another.”
The changes in the top PbTiO3 layer resulting from equilibration with the atmosphere are confirmed by x-ray experiments showing a new surface structure and by ab initio calculations based on density functional theory. “We think that the top layer is losing one out of every four oxygens at low oxygen pressure,” Stephenson said.
That discovery has implications both for the future design of devices based on these film structures and for the development of new applications. Although Stephenson emphasizes that “we’re still at this fundamental research stage, so the applications are not very certain or direct,” the researchers speculate that this awareness of chemical ferroelectric switching and the ability to manipulate it could lead to new types of catalysts and chemical actuators for nanoelectronics.
For now, the team plans to continue to investigate the chemical ferroelectric switching, including the use of different chemistries and the precise details of the mechanisms involved. “It’s a new thing to be able to do this chemically rather than electrically, so from a basic-science point of view, there are a bunch of interesting, potentially new phenomena to be studied,” said Stephenson. — Mark Wolverton
Contact: G.B. Stephenson stephenson@anl.gov
See: R.V. Wang, D.D. Fong, F. Jiang, M.J. Highland, P.H. Fuoss, Carol Thompson, A.M. Kolpak, J.A. Eastman, S.K. Streiffer, A.M. Rappe, G.B. Stephenson, “Reversible Chemical Switching of a Ferroelectric Film,” Phys. Rev. Lett. 102, 047601 (2009). DOI: 10.1103/PhysRevLett.102.047601
Work was supported under Contract No. DEAC02-06CH11357 between UChicago Argonne LLC and the Department of Energy. A.M.K. and A.M.R. acknowledge the support of the Department of Energy under Grant DE-FG02-07ER15920, the Air Force Office of Scientific Research under Grant FA9550-07-1-0397, and a Challenge Grant from the HPCMO of the Department of Defense. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.