Scientists using the U.S. Department of Energy’s Advanced Photon Source at Argonne and Stanford Synchrotron Radiation Lightsource at SLAC National Accelerator Laboratory, and the European Synchrotron Radiation Facilityin France, have found that the bacterium Cupriavidus metallidurans catalyses the biomineralisation of gold by transforming toxic gold compounds to their metallic form using active cellular mechanism, the first direct evidence that bacteria are actively involved in the cycling of rare and precious metals.
Researchers have previously reported the presence of bacteria on gold surfaces, but have never clearly elucidated the role of bacteria. Now, an international team of scientists has found that there may be a biological reason for the presence of these bacteria on gold-grain surfaces. “A number of years ago we discovered that the metal-resistant bacterium Cupriavidus metallidurans occurred on gold grains from two sites in Australia,” said Frank Reith of the University of Adelaide (Australia), leader of the scientific team. “The two sites are 3500-km apart, in southern New South Wales and northern Queensland, so when we found the same organism on grains from both sites, we thought we were onto something. It made us wonder why these organisms live in this particular environment. The results of this study point to their involvement in the active detoxification of gold complexes leading to formation of gold biominerals.”
The research showed that C. metallidurans rapidly accumulates toxic gold complexes from a solution prepared in the lab. This process promotes gold toxicity, which pushes the bacterium to induce oxidative stress and metal resistance clusters—as well as an as yet uncharacterized gold-specific gene cluster—in order to defend its cellular integrity. This leads to active biochemically-mediated reduction of gold complexes to nanoparticulate, metallic gold, which may contribute to the growth of gold nuggets.
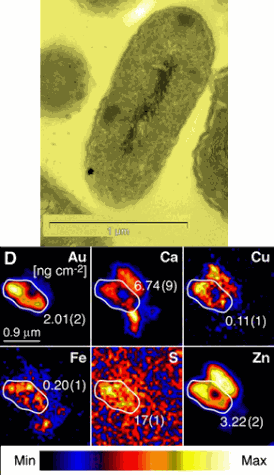
The accompanying image shows maps of pure gold with other elements. By determining what elements there are, scientists can see where the gold is located in relation to the cells.
For this study, the scientists combined synchrotron techniques used at three x-ray facilities—the X-ray Operations and Research 2-ID-D beamline at the Advanced Photon Source, the ID22N beamline at the European Synchrotron Radiation Facility, and the Stanford Synchrotron Radiation Lightsource beamline 7-3—with molecular microbial techniques to understand the biomineralisation in bacteria. It is the first time that these techniques have been used in the same study, so Reith brought together a multinational team of experts in both areas for the success of the experiment. The team was made up of scientists from Australia, Belgium, Canada, France, Germany, and the U.S.
This is the first direct evidence that bacteria are actively involved in the cycling of rare and precious metals, such as gold. The research team’s results open the door to the production of biosensors. “The discovery of an Au-specific operon means that we can now start to develop gold-specific biosensors, which will help mineral explorers to find new gold deposits,” said Reith. “To achieve this we need to further characterize the gold-specific operon on a genomic as well as proteomic level. If this research moves ahead I believe we can produce a functioning biosensor within three to five years.”
See: “Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans,” Frank Reitha,b*, Barbara Etschmannc,d,e, Cornelia Grossef, Hugo Moorsg, Mohammed A. Benotmaneg, Pieter Monsieursg, Gregor Grassh, Christian Doonani, Stefan Vogtj, Barry Laij, Gema Martinez-Criadok, Graham N. Georgel, Dietrich H. Niesf, Max Mergeayg, Allan Pringc, Gordon Southamm, and Joë l Bruggera,c, published in Proceedings of the National Academy of Sciences of the USA, published online before print October 7, 2009, doi:10.1073/pnas.0904583106
Author affiliations: aUniversity of Adelaide; bCommonwealth Scientific and Industrial Research Organisation (CSIRO) Land and Water; cSouth Australian Museum; dCSIRO Exploration and Mining, South Australian Museum; eUniversity of Tasmania; fMartin-Luther-Universität; gInstitute for Environment, Health and Safety; hUniversity of Nebraska-Lincoln; iUniversity of California, Los Angeles; jArgonne National Laboratory; kEuropean Synchrotron Radiation Facility; lUniversity of Saskatchewan; mUniversity of Western Ontario
Correspondence: *frank.reith@csiro.au
Thanks to Barrick Gold and Newmont Gold; the Natural Sciences and Engineering Research Council, Canada, which supported research at the University of Saskatchewan; P. Self, L. Waterhouse, and L. Green at Adelaide Microscopy; and J. Parson from Prophet Gold Mine for access and support. This work was supported by the Australian Research Council and Australian Synchrotron Research Funding Schemes.
The original ESRF press release can be found here.
Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. The Stanford Synchrotron Radiation Lightsource is a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.