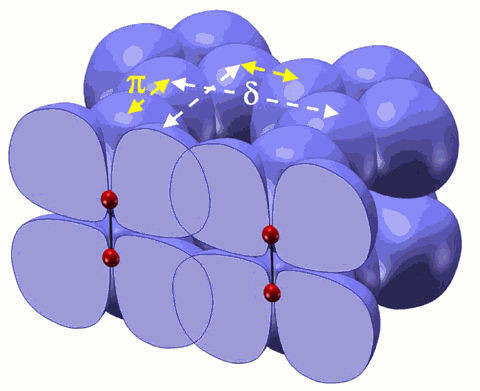
Oxygen, the third most abundant element in the cosmos and essential to life on Earth, changes its forms dramatically under pressure, transforming to a solid with spectacular colors. Eventually it becomes metallic and a superconductor. The underlying mechanism for these remarkable phenomena has been fascinating scientists for decades, especially the origin of the recently discovered molecular cluster (O2)4 in the dense, solid, red oxygen phase. Now, researchers using a recently developed synchrotron technique—high-pressure inelastic x-ray scattering—at the High Pressure Collaborative Access Team (HP-CAT) beamline 16-ID-D and the GeoSoilEnviroCARS beamline 13-ID-C,D, both at the U.S. Department of Energy’s Advanced Photon Source at Argonne National Laboratory, found that under pressure, the oxygen molecules interact through their outermost electron clouds or “1πg* orbitals.” This interaction increases as ever-greater pressure is applied, leading to electron delocalization and, ultimately, intermolecular bonding that brings four oxygen molecules together to form the (O2)4 clusters at a pressure about 100,000 times atmospheric pressure (10 gigapascals). The study was published in the Proceedings of the National Academy of Sciences. This work also demonstrates how new research tools can be used to probe subtle interactions between atoms and molecules at high pressure, potentially leading to new materials and technologies.
The researchers, from the Carnegie Institution’s Geophysical Laboratory (GL), the University of Chicago, the University of Saskatchewan, and the National Synchrotron Light Source, found that the interaction of these half-filled orbitals increases as greater and greater pressure is applied. This changes the location of the orbitals, and brings the four oxygen molecules together to form the (O2)4 clusters.
“The molecular interaction in oxygen revealed by this study is due to the unique fact that oxygen’s outmost orbital is half-filled with two unpaired electrons,” explained Yue Meng of the GL and HP-CAT and lead author of the study. “As the molecules are squeezed into smaller volumes at high pressure, electrons in the orbital inevitably move about, trying to pair with electrons in the neighboring molecules.”
To study the dense solid phases of oxygen, the researchers applied the high-pressure inelastic x-ray scattering technique, which uses a synchrotron x-ray beam to probe the electronic bonding change as a diamond anvil cell subjects a sample to many hundreds of thousands of atmospheres. The researchers combined their experimental results with theoretical calculations by collaborators from the University of Saskatchewan to further reveal that there is an increasing interaction between the neighboring (O2)4 clusters in the red-colored oxygen, providing a mechanism for forming new bonding between the oxygen clusters in still higher pressure phases.
“The behavior of oxygen at high pressure demonstrates one of the most profound effects of pressure on matter, which transforms the colorless air we breath into colorful dense solids,” continued Meng. “The drastic change in the appearance of this familiar gas is due to the bonding changes in oxygen induced by high pressure.”
“This is the first demonstration of how new tools can be used to probe the subtle interactions between atoms and molecules that lead to the formation of entirely new crystal structures,” said Russell J. Hemley, GL Director. “These new structures may give rise to entirely new electronic, magnetic, and other physical properties that could lead to new technologies.”
The formation of molecular clusters through the anti-bonding orbital called π* is well known in organic chemistry, and the electron delocalization in cluster orbitals provides several potentials for technical applications. “It is exciting to find that oxygen forms molecular clusters under high pressure through a similar mechanism,” Meng said.
Contact: Yue Meng, ymeng@hpcat.aps.anl.gov
See: Yue Meng, Peter J. Eng, John S. Tse, Dawn M. Shaw¶, Michael Y. Hu, Jinfu Shu, Stephen A. Gramsch, Chichang Kao, Russell J. Hemley, and Ho-kwang Mao, “Inelastic x-ray scattering of dense solid oxygen: Evidence for intermolecular bonding,” Proc. Nat. Acad. Sci. USA 105(33), 11640 (August 19, 2008). DOI: 10.1073_pnas.0805601105
The original press release can be found here.
GeoSoilEnviro Consortium for Advanced Radiation Sources (GSECARS) is supported by the Department of Energy (DOE)–Basic Energy Sciences (BES)–Geosciences, National Science Foundation (NSF)–Division of Earth Sciences (EAR), and the State of Illinois. The High-Pressure Collaborative Access Team facility is supported by DOE–BES, DOE–Office of Nuclear and National Security Information (Carnegie DOE Alliance Center), NSF, Department of Defense–Tactical Army Command, and the W. M. Keck Foundation. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. Argonne is a U.S. Department of Energy laboratory managed by UChicago Argonne, LLC.