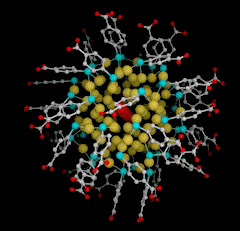
A team of researchers led by Roger Kornberg have acquired new information that will accelerate the development of practical applications for monolayer-protected gold nanoparticles, an area of intense research activity. The group, working at Stanford University, the General Medicine and Cancer Institutes Collaborative Access Team facility at the Advanced Proton Source (APS) at Argonne National Laboratory, the Stanford Synchrotron Radiation Laboratory, and the Advanced Light Source, succeeded in synthesizing and visualizing a pure crystal of gold nanoparticles coated with the thiol p-MBA (p-mercaptobenzoic acid). (Thiols, also called mercaptans, are organic compounds that have a SH sulfhydryl functional group.) The study was featured on the cover of Science magazine.
The achievement is noteworthy on several levels. First, the researchers developed a procedure for synthesizing stable clusters of a uniform size (precisely 102 gold and 44 p-MBA atoms each). Second, they determined the structure of a monolayer-protected nanocluster to the atomic level (1.1-Å resolution), and in doing so addressed long-standing questions of how sulfur binds to gold.
“Gold102 will generate (in fact it is already generating) a wide range of theoretical and experimental studies that will translate into biomedical and electronic applications in the near future,” said Guillermo Calero, a coauthor of the study published in Science. Overall, the work represents a major advance in synthesizing monolayer-protected clusters and determining the principles that guide self-assembly.
The structure of the crystallized gold-thiol cluster displays several intriguing and unexpected features. Previous studies suggested that stable clusters would have a “magic number” of gold atoms resulting from the closure of a geometric shell—forming, for example, icosahedral clusters of 55, 147, or 309 atoms when crystallized. Another view anticipated that the core would be amorphous or quasi-molten. In contrast, the gold102 cluster has a well-ordered but chiral structure that was confirmed by screening fifteen crystals derived from multiple nanoparticle preparations. Here it seems that electronic shell closing provides a better explanation for the uniform size of these clusters: in theory, each of the 44 sulfur atoms attracts a gold electron into a localized orbital, leaving 58 gold atoms, each contributing one valence electron, to form a filled shell.
The inner 49 atoms form a decahedron that is capped by two 20-atom poles arranged in C5 symmetry, yielding a total of 89 atoms that display a fivefold symmetry. The cluster’s chirality results from an asymmetric 13-atom band on the equator.
The gold-sulfur binding mode in such self-assembling nanoparticles had long been debated. One standard model had predicted each sulfur atom would bind to two adjacent gold atoms. In the gold102 crystal, however, the sulfur and gold atoms bind in a bridge conformation: each sulfur binds two gold atoms with one of the gold atoms binding two sulfurs, forming a staple-like motif. The cluster has a nucleus of gold atoms that do not contact sulfur, those in the next layer bind one sulfur, and those in the outer shell bind two sulfurs. The protective monolayer is further stabilized by interactions between the phenyl rings of the p-MBA molecules.
The Gold102 cluster, said Calero, is notably more stable than other larger clusters. The researchers are currently engaged in extending their work by the synthesis, crystallization and x-ray analysis of new gold nanoclusters with different sizes and protective monolayers.
In their initial exploration of the structure of the gold102 crystal, the researchers, from the Stanford University School of Medicine and the University of Colorado, Boulder, collected their atomic-resolution dataset using the 23-ID-B beamline of GM/CA-CAT at APS at around 1.4 Å. According to Calero, experience and better quality crystals enabled a 1.1-Å-resolution dataset at the Stanford Synchrotron Radiation Laboratory. Subsequent to the Science paper, they have been collecting data at GM/CA-CAT at an ultra-high resolution of 0.75 angstrom in order to determine the role of the sulfhydryl proton in the sulfur-gold bond, work that is still underway.— Carol Hart (twoharts@verizon.net)
See: Jadzinsky, P.D., G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg*, Structure of a Thiol Monolayer–Protected Gold Nanoparticle at 1.1 Å Resolution. Science 318, 430 (19 October 2007). DOI: 10.1126/science.1148624
Contact: *kornberg@stanford.edu
GM/CA CAT at the Advanced Photon Source has been funded in whole or in part with federal funds from the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Sciences (Y1-GM-1104). Use of the Advanced Photon Source was supported by DOE, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357. Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory (SSRL), a national user facility operated by Stanford University, on behalf of the U.S. Department of Energy (DOE), Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by DOE, Office of Biological and Environmental Research, and by NIH, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. Portions of this research were conducted at the Advanced Light Source, a national user facility operated by Lawrence Berkeley National Laboratory, on behalf of the DOE, Office of Basic Energy Sciences. The Berkeley Center for Structural Biology is supported in part by DOE, Office of Biological and Environmental Research, and by NIH and the National Institute of General Medical Sciences.
Argonne National Laboratory brings the world's brightest scientists and engineers together to find exciting and creative new solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America 's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.