The study of phonons (the quantum mechanical vibrational motion with particle-like properties found in the atomic lattice of solids) has traditionally been the province of physicists. But research carried out at the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne has confirmed a link between the existence of phonons in biological proteins and biological function, showing that the physical concept of phonons is useful in biology, too. This breakthrough was made possible by application of the inelastic x-ray scattering (IXS) technique at two APS x-ray beamlines, one of which, the new High Resolution Inelastic X-ray Scattering spectrometer (HERIX), promises to move the IXS technique into exciting new realms.
Since it went online for users early in 2007, the HERIX spectrometer at the X-ray Operations and Research 30-ID beamline at the APS has provided advances in IXS techniques, our best window into collective excitations, molecular vibrations, and elastic scattering phenomena on the atomic scale. HERIX boasts an energy resolution as fine as 1.6 meV and a wave vector transfer range as large as 72 nm-1, featuring up to nine analyzers in a parallel arrangement.
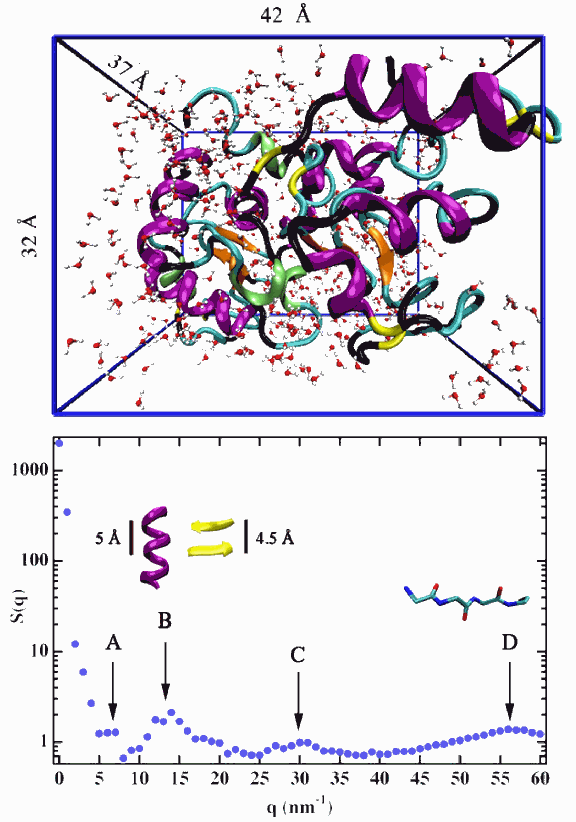
A group of researchers from MIT, the University of Florence, and Argonne National Laboratory recently exploited these capabilities of HERIX in conjunction with the nuclear resonant inelastic x-ray scattering (NRIXS) capabilities of the X-ray Operations and Research 3-ID beamline to study one of the most frequent subjects of IXS studies: the quanta of lattice vibrations known as phonons. But unlike the inorganic solids and semiconductors that are usually the subject of such studies, these phonons were found within an unusual and unexpected place: the interior of two hydrated globular proteins.
The experimenters were particularly interested in the excitations in these proteins as they manifested themselves above and below the dynamic transition temperature (TD) of 220K. (This is also known as the glass transition temperature, since below it the protein becomes more glass-like [i.e., solid], with considerable disorder and higher harmonic vibrational frequencies.) In this glass-like state, a protein molecule cannot perform large amplitude and slow molecular motions (flexibility), which are crucial for biological functions such as enzymatic activities.
The two proteins studied in the experiment were lysozyme (LYZ) and bovine serum albumin (BSA), respectively an enzyme and a transport protein. The choice of these particular proteins was partly due to their commercial availability and because large samples are needed for the IXS studies, but also, as team leader Sow-Hsin Chen of the Massachusetts Institute of Technology explains, “They happen to be two proteins that have a quite different internal structure, so it’s a fair representation of different classes of globular proteins.” The team examined LYZ and BSA samples using both the 3-ID IXS beamline and HERIX. “Because we used a momentum-resolved method, it can differentiate at which length scale these biologically all-important modes take place,” explains team member Ercan Alp the Argonne X-ray Science Division. “That's what is new in this work.”
Performing measurements at 170K, 220K, and 250K (below, at, and above the glass transition temperature, respectively), the researchers found a substantial decrease in phonon energy at a given Q value whenever the temperature exceeded TD. The slowing-down of molecular motions as represented by this softening of phonon energies provides a very strong correlation between the temperature-dependent motional behavior inside the proteins and their biological activity. At the higher temperatures above TD, the slower motions and decreased energy permit protein motions, such as conformational fluctuations that aren’t possible at lower temperatures, where molecular units that constitute the backbone of the protein perform higher frequency motions. Chen notes, “We find that the kind of phonon that is activated above 220K is in the region of Q-values near the first diffraction peak. When the temperature is below 220K, the protein doesn’t function as an enzyme. All its biological functions disappear.”
“It was surprising to see that above 220K the protein molecule acquires additional and identifiable vibrational modes,” Alp said. “This can be taken as a sign that the α-helices and β-sheets [of the proteins] have higher frequency motions at temperatures below the glass transition temperatures, which lowers their occupation numbers (the frequency with which they can be in these vibrational modes), preventing them from participating in biological functions that involve those motions.”
Aside from confirming that phonon activity is intimately tied into the biological activity of proteins, the team was struck by the fact that the two very different IXS methods complemented and verified each other’s results so seamlessly. “Both of the 3-ID and HERIX instruments were needed to complete the current study,” Alp emphasizes. “It’s most gratifying to see that the two dramatically different inelastic x-ray scattering methods we have developed at the APS, NRIXS and HERIX, both consistently point to the same physics.” The higher wave vector transfer range of the HERIX instrument allowed much shorter distances to be probed inside each protein, while the lower incident energy of the Sector 3 instrument allowed a higher incident flux and greater spectral efficiency. “That,” said Alp, “justifies the extraordinary effort that went into developing both of these instruments.”
Notes Chen, “For the first time, the existence of phonons in proteins is confirmed in such a way that we are also able to link that to the biological function, and identify the wavelength and duration of the phonon.” Chen and the team intend to expand the present work by examining even more and different sorts of proteins, continuing to exploit the unique capabilities of the HERIX and Sector 3 IXS facilities. — Mark Wolverton
Contact: S.-H. Chen, sowhsin@mit.edu
See: Dazhi Liu, Xiang-qiang Chu, Marco Lagi, Yang Zhang, Emiliano Fratini, Piero Baglioni, Ahmet Alatas, Ayman Said, Ercan Alp, and Sow-Hsin Chen, "Studies of Phononlike Low-Energy Excitations of Protein Molecules by Inelastic X-Ray Scattering," Phys. Rev. Lett. 101, 135501 (2008). DOI: 10.1103/PhysRevLett.101.135501
This research is supported by the Department of Energy Grant DE-FG02-90ER45429. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DEAC0Z-06CH11357. M.L., E.F., and P.B. acknowledge financial support from CSGI and MIUR and support from the EU-funded Marie-Curie Research and Training Network on Arrested Matter.
The Advanced Photon Source is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences.
The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America 's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.