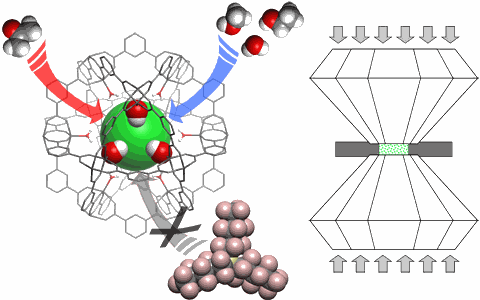
Sometimes, it’s not what’s on the outside that matters, but what happens inside, in the spaces in between. That’s certainly the case with metal-organic framework (MOF) materials, which are crystalline constructs made up of metallic ions connected to organic molecules, put together in such a way that “guest” molecules can find temporary havens within spaces and cavities inside the MOF structure. It’s a handy attribute with a wide range of possible applications, from filtering, capturing, or detecting molecules such as carbon dioxide, to storing large amounts of hydrogen in a very small space for use in fuel cells for cars. But before such practical uses can be fully realized, we need a better understanding of how MOFs react to real-world conditions outside of the controlled conditions of the laboratory. A group of researchers from the U.S. Department of Energy’s Argonne National Laboratory has investigated the behavior of a classic MOF structure under high pressures, including its compressibility characteristics, changes in morphology, and the permeability and diffusion of guest molecules. This study has important implications for the ways MOFs might be used for gas storage and other vital applications.
The Argonne experimenters, Karena W. Chapman (X-ray Science Division), Gregory J. Halder (Materials Science Division), and Peter J. Chupas (X-ray Science Division), studied a pulverized sample of Cu-btc [Cu3 (1,3,5-benzenetricarboxylate)2(H2O)3], subjected to various pressures from 0 to 8 GPa inside a diamond anvil cell (DAC), both without and with several different types of pressure-transmitting fluid. Monochromatic x-rays from the X-ray Operations and Research 1-BM-C beamline at the Argonne Advanced Photon Source provided high-pressure diffraction data to probe the behavior of the MOF lattice.
As a MOF system containing guest molecules is compressed, the guest molecules in the pressure-transmitting fluid seek out and occupy the accessible cavities inside the MOF. At low pressures, the Cu-btc MOF exhibits just this expected behavior, as the MOF structure holds up against the pressure. But as pressures inside the DAC increased, the experimenters observed a sharp transition from this “hard” regime to a “soft” regime, in which the Cu-btc framework rapidly compresses under the pressure.
The threshold pressure for this hard-to-soft transition depended on which pressure-transmitting fluid was used in the DAC gasket and occurred at higher pressures in methanol-alcohol-water (MEW) than in isopropyl alcohol. The transition pressure also varied according to the rate at which pressure was applied, beginning at higher pressure under slow compression compared to rapid compression. In the experiments performed, either without any pressure-transmitting fluid or with the use of Fluorinert (a large-molecule organic liquid), no hard-to-soft transition occurs.
This difference in the observed MOF behavior is due to the presence of smaller molecules in the alcohol-based pressure-dependent fluid that can permeate into the Cu-btc cavities. (The Fluorinert molecules are too large to enter the pores of the Cu-btc framework.) The Cu-btc MOF is able to admit a large number of these extra “guests” into the pore network of its structure to create a hypersaturated state. The guest molecules contained within the MOF framework help to counteract the external pressure on the MOF structure until a certain threshold pressure is reached, after which the MOF rapidly flexes and compresses. Beyond this threshold, the spaces within the MOF are compressed to the point where they are unable to allow any further guest molecules into the cavities. The pressure threshold depends on the size of the guest molecules; the larger the molecules, the lower the transition pressure. Smaller guest molecules also remain more mobile and able to seek out pores for longer times under pressure. The framework compressibility and flexing that occurs as the soft regime is entered is comparable to ionic solids such as NaCl. These responses of the Cu-btc MOF system to high pressure show some interesting differences when compared to those of other industrially important porous materials, such as nanoporous zeolites, that seem to be based on their differing structures.
“Just by tightening the screws on the diamond anvil cell, we raise the pressure by a few gigapascals and alter the structure as much as changing the temperature by 1000’s of Kelvin,” said Chapman. “We almost expected to see some really unusual high-pressure behaviors of these complex metal-organic framework structures."
The work of the Argonne group has provided not only a new way to peer into the behavior of MOF systems, but also hints at the practical possibilities such materials hold.
- Mark Wolverton
Contact: K.W. Chapman, chapmank@aps.anl.gov
See: Karena W. Chapman, Gregory J. Halder, and Peter J. Chupas, “Guest-Dependent High Pressure Phenomena in a Nanoporous Metal-Organic Framework Material,” J. Am. Chem. Soc. 130(32), 10524 (2008). DOI: 10.1021/ja804079z
Work performed at Argonne National Laboratory and use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. Portions of the diamond anvil cell preparation were performed at GeoSoilEnviroCARS (Sector 13), Advanced Photon Source, Argonne National Laboratory. GeoSoilEnviroCARS is supported by the National Science Foundation, Earth Sciences (EAR-0622171), and Department of Energy, Geosciences (DE-FG02-94ER14466).
Argonne National Laboratory brings the world's brightest scientists and engineers together to find exciting and creative new solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state, and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science