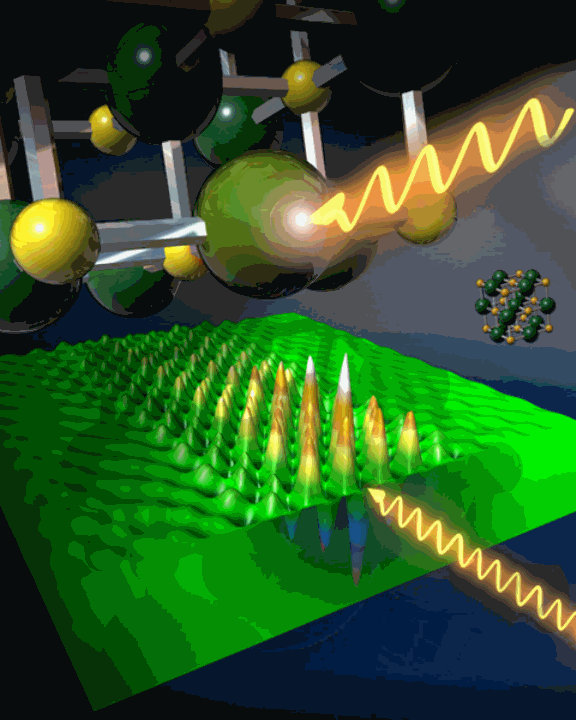
Excitons are worth getting excited about. For one thing, they are a key element in the functioning of semiconductors and insulators, and understanding their structure and how they form and behave in different materials is vitally important to the development of new materials and technologies. For another, the true nature of a particular exciton has been the subject of controversy in the materials science community for 70 years. Now, experimenters using two x-ray beamlines at the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne National Laboratory have settled the controversy, while setting the stage for research that could help improve materials used for alternative energy sources.
An exciton is a fascinating thing: an electron bound to an electron hole, acting as a single entity. But their short lifespan makes them maddeningly elusive and hard to characterize. They come in two basic varieties: the Wannier exciton, which is large, with a freely mobile, weakly-bound electron and hole; and the Frenkel exciton, smaller and very tightly bound together. Figuring out which is which in a particular material can be a challenge, because some excitons can show qualities of both types.
The controversy that has been simmering for 70 years concerns the true nature of the exciton in alkali halide insulators, the type of material in which excitons were first observed. Some scientists insisted that this particular exciton must be of the Wannier variety, while others made an equally strong case for the Frenkel type. Eventually a consensus formed around the idea that the alkali halide exciton is some kind of “in between” variety. But limitations in experimental methods made it impossible to settle the issue until a group of experimenters from the University of Illinois at Urbana-Champaign (UIUC), The University of Chicago, Tamkang University, the University of Pierre and Marie Curie, the Synchrotron Soleil, and Brookhaven National Laboratory nailed down the alkali halide exciton once and for all, using the inelastic x-ray scattering (IXS) technique to examine the time-dependence structure of the elusive quasiparticle.
The researchers examined the valence charge-transfer exciton in LiF with causally-inverted IXS at the ChemMatCARS 15-ID-B,C,D beamline and the XOR/CMC 9-ID-B,C beamline at the APS, achieving a time resolution of 20.67 attoseconds and a spatial resolution of 0.533 angstroms. “The output of our measurement is basically a time-dependence of the structure of this exciton,” said the study co-author Peter Abbamonte of the Frederick Seitz Materials Research Laboratory at UIUC. Using mathematical methods that impose causality as a constraint, the team was able to image excitons in real time and determine whether they are of the Wannier, Frenkel, or an intermediate variety.
Initially, they found that while the LiF exciton has a large binding energy, implying a Frenkel model, it is also dispersive in nature, unlike the tightly bound Frenkel. But as the experimenters further isolated the exciton’s time structure, this dual personality began to disappear. Instead, they found that the LiF charge-transfer exciton displays a rigid structure, staying only within a two unit-cell region. “It turns out it’s a textbook Frenkel exciton,” Abbamonte said, as definitively identified by its rigidity and relatively high binding energy.
But how can the LiF exciton indulge in charge transfer if it’s pretty much stuck in one place in the atomic lattice? Abbamonte acknowledged, “That was actually the original claim, that a charge transfer exciton cannot be a Frenkel exciton, because [a charge transfer exciton] can’t be localized.” But the team’s results show that the LiF exciton actually resides in what can be considered a “superatom” that encompasses several different sites, all of which can still be defined with Wannier functions. This is the key to the LiF exciton’s two-faced personality that has baffled scientists for decades. The fact that its rigid structure doesn’t change over its brief life defines it as a Frenkel exciton, but its habit of parking itself over two different unit cells gives it a Wannier-like mask.
Settling this question of the nature of alkali halide excitons has implications beyond merely ending a long-standing scientific argument. The researchers have also demonstrated that Frenkel excitons can be described with a relatively simple mathematical model, unlike their Wannier-type brethren, which can require supercomputer power to model properly. As Abbamonte explained, “That’s actually important, because it lets you scale up your calculations to big macroscopic dimensions,” meaning the world of practical applications. Because Frenkel excitons are found not just in insulators but in other handy materials such as organic crystals, a better understanding and description of these quasiparticles might soon help to create highly efficient solar cells and other devices. – Mark Wolverton
Contact; P. Abbamonte, abbamonte@mrl.uiuc.edu
See: Peter Abbamonte, Tim Graber, James P. Reed, Serban Smadici, Chen-Lin-Yeh, Abhay Shukla, Jean-Pascal Rueff, and Wei Ku, “Dynamical reconstruction of the exciton in LiF with inelastic x-ray scattering,” Proc. Nat. Acad. Sci. USA, 105 (34), 12159 (2008). DOI:10.1073/pnas.0801623105
IXS measurements were supported by Department of Energy (DOE) Grant DE-FG02-07ER46459 through the Frederick Seitz Materials Research Laboratory. ChemMatCARS is supported by the National Science Foundation/DOE Grant CHE-0535644. The DuPont-Northwestern-Dow Collaborative Access Team is supported by E.I. DuPont de Nemours & Co., The Dow Chemical Company, and the State of Illinois. W.K. was supported by DOE Grant DE-AC02-98CH10886 and DOE-Computational Materials Science Network. C.-L.Y. was supported by NSC Grant 95-2112-M-032-001 and the Taiwanese National Science Council Research Abroad Program. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory brings the world's brightest scientists and engineers together to find exciting and creative new solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America 's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.