A molecular enzyme that is involved in many necessary and beneficial cellular processes would seem to be a friend best left to go about its business. But when a defective or overactive variation of that same enzyme, called calpain, is linked to a startling array of illnesses, then finding out how to regulate calpains becomes an important research objective. Scientists using x-ray beams at three U.S. Department of Energy national research facilities, including the Advanced Photon Source at Argonne National Laboratory, have gained new insights into regulation of calpain that could lead to the development of new drugs to restrain calpain’s less beneficial tendencies.
As molecular overachievers, calpains play a role in many cellular processes, including the movement of cells in tissues, the death of damaged cells, insulin secretion, and brain cell and muscle function. The downside of this broad set of responsibilities is that defective or overactive calpains have been linked to an array of disorders, including a form of muscular dystrophy, Type 2 diabetes, gastric cancers, Alzheimer’s and Parkinson’s diseases, cataracts, and the death of both heart muscle in heart attacks and of brain tissue in stroke and traumatic brain injury.
“Our basic findings on calpain regulation could add useful pieces to the puzzles of these disorders and perhaps reveal targets for drugs to treat them,” said Douglas Green, of the St. Jude Department of Immunology and senior author of the Nature article on the calpain study.
Calpains are triggered by calcium flowing into a cell. This process induces the enzyme to snip apart many target proteins, as part of the cell’s regulatory machinery. However, such a critical enzyme needs ultra-precise control, which is the job of another protein called calpastatin. A central question has been how calpastatin is so exquisitely specific in attaching to calpain and inhibiting it—essentially ignoring other highly similar enzymes in the cell.
“Previous studies on calpastatin had revealed how a few of the parts of the calpastatin molecule attach to calpain in the inhibition process,” said Green. “However, there was no overall picture of calpastatin that revealed how it was so precise in its attachment and potent in its function.”
To obtain that overall picture, St. Jude researchers and a colleague from McGill University employed the x-ray crystallography technique at Southeast Regional Collaborative Access Team beamlines 22-BM-D and 22-ID-D at the Advanced Photon Source, beamlines 5.0.1-3 at the Advanced Light Source (Lawrence Berkeley National Laboratory), and beamline X29 at the National Synchrotron Light Source (Brookhaven National Laboratory). To overcome the crystallization bottleneck, a lengthy and unpredictable variable in x-ray crystallography, the investigators used nuclear magnetic resonance spectroscopy to tailor the perfect enzyme-inhibitor complex.
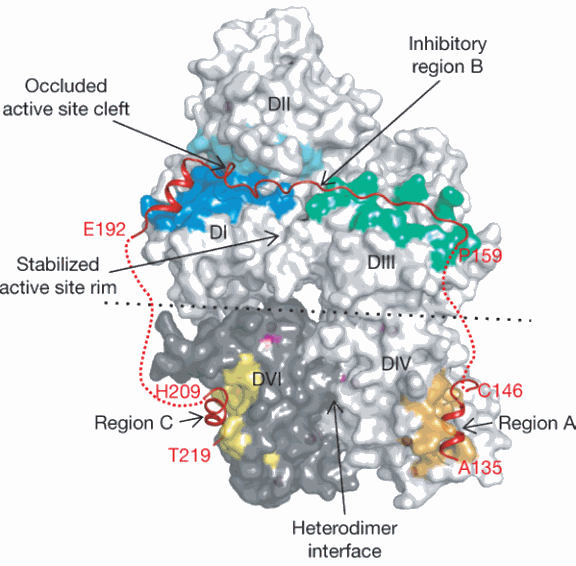
Tudor Moldoveanu, a postdoctoral fellow in Green’s laboratory, performed x-ray structural analysis on such a protein crystal that consisted of a critical part of the calpastatin molecule attached to calpain. The structural picture obtained of the two proteins clutched together clearly revealed why calpastatin so specifically attaches to calpain.
“Calpain has multiple domains, and what we saw was that calpastatin wraps itself around pretty much every domain of calpain,” said Moldoveanu. This attachment not only blocks the portion of the enzyme called the active site, where calpain performs its snipping function, but also covers regions away from that site. Such a broad molecular embrace guarantees that calpastatin will potently and rapidly shut down calpain’s function. This broad embrace also guarantees that calpastatin will precisely recognize only calpains, rather than mistakenly attach to other similar enzymes in the cell.”
Furthermore, the researchers discovered how calpastatin evades being chewed up by calpain. Calpastatin’s survival enables it to be repeatedly recycled to inhibit calpain, making it an even more effective regulator.
The researchers’ structural information also showed how calpain changes its shape once it is activated by calcium and how this transformation renders it a target of calpastatin attachment and thus inhibition.
“This new structural information on calpastatin and on calpain’s conformational changes not only explains a lot about calpain’s regulation,” Green said. “It also gives us information we can use to develop targets for drugs that could either activate or inhibit calpain.”
Contact: Douglas R. Green douglas.green@stjude.org
See: Tudor Moldoveanu, Kalle Gehring, and Douglas R. Green, “Concerted multi-pronged attack by calpastatin to occlude the catalytic cleft of heterodimeric calpains,” Nature 456, 404-408 (20 November 2008). DOI:10.1038/nature07353
Funding for this research was provided by the National Institutes of Health, the National Cancer Institute, the Canadian Institute of Health Research, and the American Lebanese Syrian Associated Charities.
SER-CAT is funded mainly through state legislative funds, agencies, and individual member universities at the university, department, or individual research group levels. SER-CAT is operated by the University of Georgia. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America 's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The original news release can be found here.