The search for ways to conserve energy is leading scientists to explore unexpected but important avenues, such as technologies that make extensive use of alloys that are subject to corrosion, which can result in significant energy inefficiency. Researchers from the U.S. Department of Energy’s (DOE’S) Argonne National Laboratory, using three DOE facilities at Argonne, and a variety of experimental techniques, have studied the phase composition of oxide scale that protects alloys. Their research indicates that a change in the phase composition of the oxide scale on alloy surfaces could save over $1 billion per year in lost energy for the U.S. hydrogen industry alone.
Structural alloys are generally protected from extensive corrosion by oxide scales that develop on the alloy surface at high temperatures. The diffusion rate of carbon in oxides, such as those expected to comprise the majority of these scale layers, is negligible. Despite this fact, carbon often diffuses into alloys and leads to brittleness and even pitting corrosion. Carbon transport through the oxide scale is usually considered to involve the diffusion of carbon-bearing molecules such as CO and/or CO2 through pores or cracks in the scales. But this mechanism has several fundamental flaws that suggest an alternative mechanism must be at play.
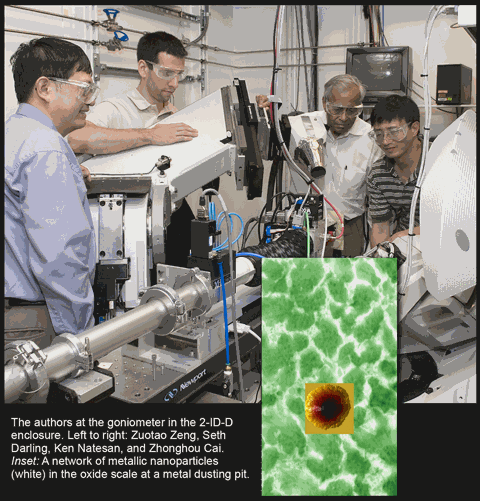
The Argonne research team of Zuotao Zeng and Ken Natesan (Nuclear Engineering Division), Zhonghou Cai (X-ray Science Division), and Seth Darling (Center for Nanoscale Materials, CNM) devised an alternate explanation for the corrosion mechanism. It is well known that, unlike in oxides, carbon can dissolve in and diffuse through nickel and iron metals. Therefore, if metal particles are present in oxide scale, a new path for carbon atom transport is available that does not involve defects in the scale. The Argonne team probed oxide scales using nanobeam x-ray analysis at X-ray Operations and Research beamline 2-ID-D at the Advanced Photon Source, magnetic force microscopy at the CNM, and scanning electron microscopy at the Argonne Electron Microscopy Center for Materials Research. Their results, which were published as an article in Nature Materials, show that metal nanoparticles are indeed present in the scale. These metal nanoparticles join to form continuous channels for carbon transfer from the exposure environment to the substrate alloy.
Traditional x-ray beams are too large to analyze the cross section of oxide scales that are only a few micrometers in thickness. Further complicating the problem, some alloys develop scales with several sublayers. Local phases could be analyzed by transmission electron microscopy, but it is difficult to prepare samples for this technique that allow us to study the regions of interest. Moreover, such preparations could also induce decomposition of oxides to metal.
These technical problems are some of the reasons that the local chemistry of oxide scales on alloy surfaces has not been carefully studied to date. In contrast with transmission electron microscopy, the nanobeam x-rays from the APS and the tip of the CNM magnetic force microscope can easily scan through the whole oxide scale and achieve the information of phases and oxidation state of elements.
It is projected that these new Argonne alloys could be used to build facilities that can recycle the wasted high-temperature heat and save more than $1 billion in lost energy for the U.S. hydrogen industry alone, based on the current cost of the natural gas needed to produce an amount of energy equivalent to that lost during hydrogen production.
This study may have a broad influence on not only metal dusting and carburization, but also in other research areas such as alloy development and surface coatings for high-temperature fuel cell applications.
Read the Chicago Tribune article on this research.
(Note: For information on licensing this technology, please contact Stephan Lake (slake@anl.gov) in the Argonne Office of Technology Transfer.)
See: Z. Zeng, K. Natesan, Z. Cai, and S.B. Darling, “The role of metal nanoparticles and nanonetworks in alloy degradation,” Nat. Mater. 7, 641 (2008). DOI:10.1038/nmat2227
Contact: K. Natesan, natesan@anl.gov
This work is supported by the US Department of Energy, Office of Industrial Technologies. Use of the Advanced Photon Source, the Center for Nanoscale Materials, and the Electron Microscopy Center for Materials Research were supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory brings the world's brightest scientists and engineers together to find exciting and creative new solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership, and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.