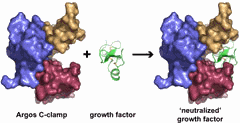
Argos is a fruit fly protein that acts as a “decoy” receptor, binding growth factors that promote the progression of cancer. Researchers from the University of Pennsylvania School of Medicine (UPSM), using x-ray beamlines at two U.S. Department of Energy synchrotron light sources, have shown how Argos achieves this feat. Knowing how Argos then neutralizes tumor growth may lead to new drug designs for inhibiting cancer. The study appeared online in the journal Nature.
Many types of tumors grow because of over-expression of a protein known as the epidermal growth factor receptor (EGFR), or a peptide hormone called epidermal growth factor (EGF) that binds and activates EGFR. Argos mimics EGFR by binding to EGF. But, unlike EGFR, Argos does not signal cells to grow.
In theory, surmise the researchers in this study, a drug designed to resemble Argos could bind cancer growth factors and prevent them from signaling cancer cell growth. The investigators previously found that Argos works this way in the fruit fly, binding and neutralizing the fly version of EGF called Spitz. Inhibition of Spitz in this way is crucial for proper development of the fly eye.
“There are several ‘designer’ cancer drugs that fight tumors driven by EGFR-like receptors, such as Herceptin, Erbitux and Tarceva,” says lead author Mark A. Lemmon, Professor of Biochemistry and Biophysics at UPSM. “Whereas these drugs all attack the receptor itself, an Argos-like drug would instead neutralize the cancer growth factor by mimicking a silent receptor. This is a change in paradigm for tumor-growth inhibition in this class of cancers.”
Approaches using molecules that neutralize growth factors have proven themselves in other cases. The Avastin antibody works well to block the molecule that activates the vascular endothelial growth factor receptor and several drugs can block tumor necrosis factor-a in arthritis, including Remicade, Humira and Enbrel. An Argos-like drug would work the same way in EGFR signaling, suggests Lemmon.
In the current study, Lemmon and colleagues have worked out the details of the three-dimensional structure of Argos when it binds to Spitz. “We were surprised to find that Argos has three very similar domains that capture Spitz by surrounding it like a C-clamp,” explains Lemmon. Although Argos binding to Spitz mimics the characteristic binding of EGFR to EGF, Argos and EGFR do not share the same amino acid sequence or structural similarities.
The structure of Argos was determined by x-ray crystallography carried out at the General Medicine and Cancer Institutes Collaborative Access Team (GM/CA CAT) beamline 23-ID-D at the Argonne Advanced Photon Source, and at the Advanced Light Source (Lawrence Berkeley National Laboratory), beamline 8.2.1. Computer analysis was then used to put together all the data into a three-dimensional projection of the growth factor and its binding molecule.
The next step is to identify a human protein that is similar in structure to Argos. Intriguingly, Argos shares structural similarities with the human receptors for transforming growth factor b and the urokinase plasminogen activator. “There are quite a few other human proteins with similar predicted structures whose function is not yet known,” says Lemmon. “We think that one of these might be a functional analogue of Argos, and we could exploit that as a drug.”
Contact: Mark A. Lemmon, mlemmon@mail.med.upenn.edu).
See: Daryl E. Klein, Steven E. Stayrook, Fumin Shi, Kartik Narayan, and Mark A. Lemmon, “Structural basis for EGFR ligand sequestration by Argos,” published in Nature, advance online publication 25 May 2008 | doi:10.1038/nature06978.
The original news release can be found here.
This work was supported by grants from the National Institutes of Health (to M.A.L.) and the US Army Breast Cancer Research Program (to D.E.K. and M.A.L.).
Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. The GM/CA CAT has been established by the National Institutes of Health's (NIH) National Institute of General Medical Sciences (NIGMS) and National Cancer Institute (NCI) to build and operate a national user facility for crystallographic structure determination of biological macromolecules by X-ray diffraction.
Argonne National Laboratory brings the world's brightest scientists and engineers together to find exciting and creative new solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America 's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.