Samples of kerogen (oil precursors) and coal can be persuaded to give up the secrets of their origin, thanks to a new application of a standard synchrotron x-ray technique employed by researchers from ExxonMobil using the XOR/CMC 9-BM beamline at the Argonne Advanced Photon Source. Characterizing and comparing the sulfur content and form in kerogen and coal can reveal the origin of a sample, as well as the thermal age of the sample and the process by which the sample was derived from organic matter. This technique is sure to be of great interest for those engaged in the search for fossil fuels.
All sedimentary organic matter and fossil fuels contain sulfur in varying quantities and diverse forms. A basic understanding of sulfur chemistry in these materials is extremely important for predicting their reactivity and physical properties. Organic sulfur in kerogens and coals may be derived directly from sulfur-containing biomolecules or, more likely, from sulfide incorporated during the early stages of its conversion from sediment into kerogen (diagenesis). Some sulfate-reducing bacteria live without oxygen and reduce sulfate (instead of oxygen) as a means of oxidizing organic matter during diagenesis. However, knowledge about the chemical forms initially introduced into sedimentary organic matter and subsequent sulfur transformations pathways during the maturation of kerogen (the insoluble hydrocarbon in sedimentary rock) and the conversion of terrestrial plant material into coal (or coalification) come from indirect evidence. Indirect methods employ decomposition of the organic solid followed by separation and identification of evolved liquid and gaseous products. Incomplete decomposition of the solid and undetected chemical transformations that occur during decomposition are inherent limitations of this approach. The synchrotron x-ray technique sulfur x-ray absorption near-edge structure (S-XANES), a refinement of the standard XANES technique, provides a way to directly identify and quantify sulfur forms in complex solids containing carbon. By characterizing and comparing kerogen, unaltered peats, lignites, coals, and pyrolyzed peats using S-XANES, it is possible to directly identify thermal chemistry transformations involving sulfur and non-thermal chemical pathways for coalification and kerogen maturation.
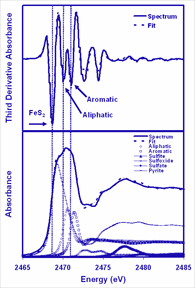
Well-defined kerogens that span a wide range of organic matter types and maturities were identified and gathered by ExxonMobil, IFP, and the University of Utah and analyzed using the S-XANES technique at the XOR/CMC beamline to quantifiy the chemical forms of sulfur in kerogens. The S-XANES analysis starts by collecting the sulfur x-ray absorbance spectrum of the sample at beamline 9-BM configured specifically for low-energy operation, taking the third derivative of that spectrum, and curve resolving the third derivative spectrum with a non-linear least squares regression program using third derivative spectra of model compounds. The third derivative method is mathematically less susceptible to fitting false minima than direct fits of the absorption spectrum. Also, the model spectra are not always representative of the sample with respect to broad multiple scattering features of the absorption spectrum. This method de-emphasizes broad multiple scattering features during the fitting process. This is illustrated, in the accompanying figure, for a Type II kerogen. Fits of the S-XANES third derivative of absorbance and absorbance spectra to model compound data reveal the chemical nature of both organic and organic sulfur forms. An inverse correlation between the relative amount of aliphatic sulfur and the amount of aromatic carbon was discovered for non-oxidized organic sulfur forms. This generalization is independent of organic matter type and the total amount of organic sulfur present. The cleavage of weak aliphatic sulfur bonds is thought to promote early petroleum generation during the maturation of high sulfur kerogens. These findings have been reported in Energy and Fuels [1].
Well-defined peats were gathered that have diverse organic matter inputs and depositional settings and were analyzed by researchers from ExxonMobil and the University of South Carolina. S-XANES analysis revealed that peat contains aliphatic sulfur and SO3 groups with smaller amounts of aromatic sulfur. Aliphatic sulfur is present as mercapto, disulfide and aliphatic sulfide. Peats were pyrolyzed to increasing severity and analyzed using S-XANES. The total amount of organic sulfur in thermally stressed peat is comparable to that in low-sulfur lignites and higher-rank coals indicating that much of the organic sulfur in lignites and higher-rank coals is derived from sulfur species incorporated during peatification (early diagenesis). The level of aromatic sulfur increases as the severity of peat pyrolysis increases. The relative increase is due to their formation from aliphatic sulfur forms and through selective loss of disulfide, aliphatic sulfide, and SO3 species. When peat is thermally stressed to a state equivalent to the bituminous stage of coalification , aromatic sulfur is the dominant organic sulfur form. These findings have been reported in Energy and Fuels [2].
References
[1] S.R. Kelemen, M. Afeworki, M.L. Gorbaty, M. Sansone, P.J. Kwiatek, C.C. Walters, H. Freund, M. Siskin, A.E. Bence, D.J. Curry, M. Solum, R.J. Pugmire, M. Vandenbroucke, M. Leblond, F. Behar, "Direct Characterization of Kerogen by X-ray and Solid-State 13C Nuclear Magnetic Resonance Methods," Energ. Fuel 21(3), 1548 (2007) . DOI: 10.1021/ef060321h
[2] S.R. Kelemen, M. Afeworki, M.L. Gorbaty, P.J. Kwiatek, M. Sansone, C.C. Walters, A.D. Cohen, "Thermal transformations of Nitrogen and Sulfur Forms in Peat Related to Coalification," Energy and Fuels, 2006, 20, 635-652.
Contacts: Simon.R.Kelemen@ExxonMobil.com and Michael.Sansone@ExxonMobil.com
Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Argonne is a U.S. Department of Energy laboratory managed by UChicago Argonne, LLC