Understanding why some mosquitoes are resistant to malaria could help fight a disease that afflicts and kills millions of people. Now, researchers from the University of Texas (UT) Southwestern Medical Center, the Howard Hughes Medical Institute, and the Institut National de la Santé et de la Recherche Médicale, using the Structural Biology Center (SBC) beamline 19-ID at the Argonne Advanced Photon Source, have found the answer.
The researchers focused on TEP1, a protein in the mosquito's immune system. When a mosquito is infected with a parasite that causes malaria, a biochemical reaction is triggered that physically transforms TEP1 into an active state capable of grabbing on to the parasite's surface and targeting it for termination.
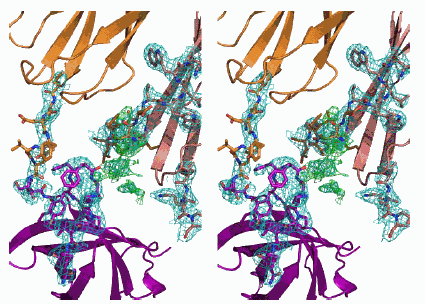
As detailed in a study appearing in the Proceedings of the National Academy of Sciences, the group employed x-ray protein crystallography at the SBC beamline to uncover TEP1's three-dimensional structure. They found that the genetic differences between mosquitoes that are resistant and those that are susceptible to the parasite mostly manifest in a region of the TEP1 protein dubbed "the warhead," the portion that grabs the malarial parasite.
"TEP1 is a scout that finds the enemy, in this case malarial parasites, then plants a homing signal on the enemy and calls in the air strike," said Richard Baxter, a postdoctoral researcher in biochemistry at UT Southwestern and lead author of the study.
Understanding how some mosquitoes can fend off malaria might some day lead to reducing or even eliminating the mosquito's capacity to transmit the devastating disease, Baxter said.
"We have been trying to cure people of malaria for over a century," said Baxter. "Only recently have people started to think about curing mosquitoes of malaria."
Nobel laureate Johann Deisenhofer, who is senior author of the study, said, "This finding opened my eyes to the fact that mosquitoes are almost as unhappy about malaria as we are. They try to get rid of it." Deisenhofer is a professor of biochemistry, an HHMI investigator, and holder of the Virginia and Edward Linthicum Distinguished Chair in Biomolecular Science. He was awarded the 1988 Nobel Prize in chemistry for using x-ray crystallography to describe the structure of a protein involved in photosynthesis.
Malaria is one of the leading causes of disease and death in the world. About 350 million to 500 million people worldwide are infected with malaria, according to the Bill and Melinda Gates Foundation. Each year more than one million die, primarily children in Africa.
About 40 percent of the world's population lives in areas with mosquitoes that carry malaria. Prevention and treatment have been hampered by cost, the rise of drug-resistant malarial parasites, and the lack of a vaccine.
Malaria is caused by parasites of the genus Plasmodium, which are spread to humans through mosquito bites. A mosquito picks up the parasite via infected human blood. The parasite then embeds itself in the mosquito’s gut wall and reproduces, eventually passing to the salivary glands. The mosquito then infects new people during subsequent bites.
The research group's French collaborators, using a Plasmodium species that infects rodents, previously determined that the gene for TEP1 occurs in two forms, or alleles. One, called TEP1r, occurs in mosquitoes that are resistant to malarial infection. Another, TEP1s, is found in mosquitoes that are vulnerable to infection.
The TEP1r and TEP1s proteins are 93% genetically identical, and the new study, in which TEP1r was structurally analyzed, shows that the differences cluster around the warhead area, Baxter said. This finding reinforces the theory that the warhead is a key element of the overall immune response to malaria in mosquitoes.
In future studies, the researchers will genetically manipulate the warhead to study its binding properties. In addition, further research is needed to determine what other elements of the mosquito’s immune system are activated once TEP1 binds to an invader.
Contact: johann.deisenhofer@utsouthwestern.edu
See : Richard H. G. Baxter, Chung-I Chang, Yogarany Chelliah, Stéphanie Blandin, Elena A. Levashina, and Johann Deisenhofer; "Structural basis for conserved complement factor-like function in the antimalarial protein TEP1," Proc. Nat. Acad. Sci. USA 104, 11615 ( July 10, 2007)
DOI: 10.1073_pnas.0704967104
The original press release can be found at: https://utswmed.org/.
The work was supported by The Welch Foundation. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences.
Argonne is a U.S. Department of Energy laboratory managed by UChicago Argonne, LLC