One-dimensional nanostructures have demonstrated great potential to be used in new optoelectronic devices, such as blue-light lasers for next generation optical storage media. ZnO semiconducting nanowires with the hexagonal or “wurtzite” structure,have been one of the most promising new materials because they form natural lasing cavities. As ZnO wires are alloyed with Mg to form MgxZn(1-x)O, its bandgap, which determines the color of light emitted by the las er, increases from 3.3 eV to 7.8 eV. Technologically, higher energy (bluer) light is desirable, because it can be focused more tightly, thus increasing the information that can be stored on media such as audio CDs and video DVDs. Recently, researchers from the Advanced Photon Source (APS) and University of Florida have used the time-resolved x-ray excited optical luminescence (TRXEOL) technique at X-ray Operations and Research beamline 4-ID-C at the APS to identify which atoms and structures within the wire are responsible for the visible light luminescence. Such information is essential in order to fully understand the light emission properties of complex nanosystems such as coaxial (Mg,Zn)O core-shell nanowires. Using this technique, the origin of the luminescence centers can be pinpointed by varying the energy of APS x-rays to excite either Zn, Mg and O atomic core levels, and analyzing the wavelength and lifetime of the emitted visible light photons.
One of the keys to increasing light emission efficiency is to improve the quality of the surface or interface that border the luminescent medium. Since nanostructures are so small surface defects often quench the luminescence, which drastically reduces the efficiency. By coating the core nanostructure with a higher band-gap shell it is possible to dramatically increase the light emission, provided the resulting core-shell interface does not produce a large concentration of defects.
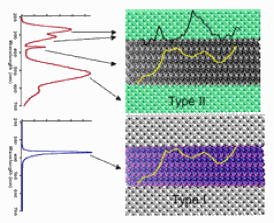
The results of this study are summarized in the figure. The TRXEOL spectra from two types of MgxZn(1-x)O core-shell nanowires are shown. The type I wire is composed of a core with a low Mg concentration wurtzite structure, encased by a higher Mg concentration sheath with the same wurtzite structure. The type II wire, on the other hand, is composed of a wurtzite structure core with no Mg dopants encased by a heavily doped MgxZn(1-x)O sheath with the cubic rock-salt structure. The luminescence spectrum from the type I wire is dominated by a very intense, short-lived, band-edge exciton peak. For the type II wire, the exciton emission is much weaker, and there is also emission from several long-lived defect levels. By monitoring the x-ray energy dependence of the various emitting optical states, particularly at the Zn L3 edge (shown), we have determined that in both types of structures the band-edge exciton state is localized in the core, while the defect levels in type II structures are localized in the sheath region. The shell in type I structures is dark (does not emit). These results should prove of great value to future nanoengineers who need to integrate such building blocks into functional nanodevices.
Contact: R. Rosenberg, rar@aps.anl.gov.
See: Richard A. Rosenberg, Gopal K. Shenoy, Matthew F. Chisholm, Li-Chia Tien, David Norton, and Steven Pearton, “Getting to the Core of the Problem: Origin of the Luminescence from (Mg,Zn)O Heterostructured Nanowires,” Nano. Lett. 7(6), 1521 (2007). DOI: 10.1021/nl0702923
Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. The work at Oak Ridge National Laboratory sponsored by the Office of Basic Energy Sciences, U.S. Department of Energy, under contract managed and operated by UT-Battelle, LLC. The work at UF was supported by the National Science Foundation (DMR0400416, 0305228, Dr.L.Hess). The authors at UF also acknowledge the Major Analytical Instrumentation Center, Department of Materials Science and Engineering, University of Florida.
Argonne is a U.S. Department of Energy laboratory managed by UChicago Argonne, LLC.