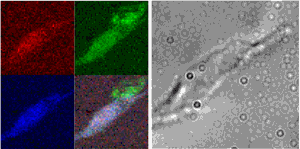
The process of growing new blood vessels from existing ones—angiogenesis—is important in growth, development, and wound healing. It is also critical to the transition that allows tumors to spread throughout the body, which has had researchers scrambling for ways to stop angiogenesis in the fight against cancer. One of the factors found to be critical to blood vessel growth is copper, a vital nutrient that plays important roles in many life processes. Inhibition of blood vessel growth can be induced by administering compounds that reduce copper in the body without disrupting other functions, and some are even in clinical trials for use in cancer therapy. Yet the biological basis for this particular sensitivity of angiogenesis to copper has been an enigma. In search of an answer, researchers from the Biosciences and X-ray Science divisions of Argonne National Laboratory, and the Department of Medicine, Section of Hematology/Oncology at The University of Chicago have used x-ray fluorescence microprobe (XFM) imaging at X-ray Operations and Research beamline 2-ID-E at the Advanced Photon Source (APS) to look at the distribution of copper in both a cell model of angiogenesis and sections of breast tumor tissue rich in blood vessels. The researchers reported (Finney et al., PNAS 104(7), 2247-2252, 2007) that cells undergoing angiogenesis exhibit a distribution of their cellular copper that is distinctly different from other cells. This discovery may help explain how copper-reducing cancer therapy works.
The group began their study by examining a model of angiogenesis that uses human microvascular endothelial cells (HMVECs) to form capillary-like structures within about eight hours of being stimulated with specific growth factors. They then examined the distribution of elements in these structures using XFM imaging resources at APS sector 2. These particular beamlines employ Fresnel zone plate optics to focus coherent x-rays to sub-micron spot sizes, through which the sample is raster scanned. By collecting emitted fluorescence spectra at each point using an energy-dispersive detector, the researchers obtained images that quantified the quantitative and spatial distribution of a variety of medium-Z elements important to biological systems. Overlaying these elemental maps onto optical images of the cells, they then correlated elemental content with cellular structures. Their findings were very clear. They observed a dramatic relocalization of between 80 to 90% of cellular copper to the tips of tendril-like projections angiogenic cells send out between one another and across the cellular membrane within the first two hours.
To extend these studies to a living organism, they then examined sections of breast tumor tissue that are rich in newly formed blood vessels. Here, too, they found that in contrast to both non-vascularized areas and areas of mature blood vessels, in areas of tissue where blood vessels were newly invading surrounding tissue, the cells showed copper localized at the periphery of the cells and in areas immediately outside of any apparent cellular structures.
These findings improve our understanding of how removing copper from the body can help stop angiogenesis. If vital copper being translocated outside of the cell during angiogenesis is intercepted by a drug, the process stops. Yet, the dynamics of cellular copper seen here also have broad implications on the regulation of the metal ion content in metal-binding proteins. If such dramatic changes in where cellular copper is being stored and used can happen so rapidly during angiogenesis, it presents a very fluid picture of the interactions of metal ions with the proteins and macromolecules that bind them in the cell.
Contact: David Glesne (dglesne@anl.gov)
See: Lydia Finney, Suneeta Mandava, Lyann Ursos, Wen Zhang, Diane Rodi, Stefan Vogt, Daniel Legnini, Jörg Maser, Francis Ikpatt, Olufunmilayo I. Olopade, and David Glesne, “X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis,” Proc. Nat. Acad. Sci. USA 104, 2247 (February 13, 2007). DOI: 10.1073/pnas.0607238104
This work and use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract DE-AC-02-06CH11357. O.I.O. is a Doris Duke Distinguished Clinical Scientist.
Argonne is a U.S. Department of Energy laboratory managed by UChicago Argonne, LLC