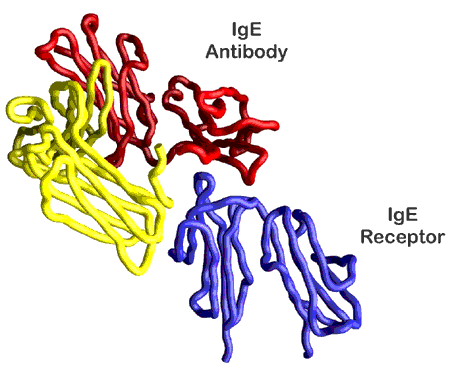
Researchers from Northwestern University and the Harvard Medical School have identified the structure of the interaction complex of two molecules, the antibody immunoglobin-E (IgE) and its high-affinity receptor, that are central to the allergic response in humans. This work was carried out APS beamline 5-ID, which is operated by the E.I. Du Pont de Nemours & Co.-Northwestern University-The Dow Chemical Company Collaborative Access Team. The discovery of how the antibody binds to the mast cell receptor could lead to the development of a new class of drugs that attack allergies at their source, preventing the cascade of released chemicals that leads to the itching, sneezing and congestion of allergies, the life-threatening respiratory distress of asthma, and anaphylactic shock. Today's commercial drugs only treat symptoms once the allergic response is already under way. The findings also may lead to novel treatments for autoimmune disorders and improved cancer therapies based on antibodies.
Scott C. Garman*, Beth A. Wurzburg*, Svetlana S. Tarchevskaya*, Jean-Pierre Kinet**, & Theordore S. Jardetzky*
*Dept. of Biochemistry, Molecular Biology and Cell Biology, Northwestern University, Evanston, IL 60208 U.S.A.
** Dept. of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215 U.S.A.
Correspondence should be addressed to: TSJ at tedj@northwestern.edu
- From "Structure of the Fc fragment of human IgE bound to its high-affinity receptor FcεRIα," Nature 406, 259 - 266 (2000) © Macmillan Publishers Ltd.